h-Channels Contribute to Divergent Intrinsic Membrane Properties of Supragranular Pyramidal Neurons in Human versus Mouse Cerebral Cortex
- PMID: 30392798
- PMCID: PMC6447369
- DOI: 10.1016/j.neuron.2018.10.012
h-Channels Contribute to Divergent Intrinsic Membrane Properties of Supragranular Pyramidal Neurons in Human versus Mouse Cerebral Cortex
Abstract
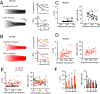
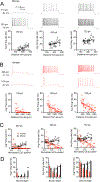
Gene expression studies suggest that differential ion channel expression contributes to differences in rodent versus human neuronal physiology. We tested whether h-channels more prominently contribute to the physiological properties of human compared to mouse supragranular pyramidal neurons. Single-cell/nucleus RNA sequencing revealed ubiquitous HCN1-subunit expression in excitatory neurons in human, but not mouse, supragranular layers. Using patch-clamp recordings, we found stronger h-channel-related membrane properties in supragranular pyramidal neurons in human temporal cortex, compared to mouse supragranular pyramidal neurons in temporal association area. The magnitude of these differences depended upon cortical depth and was largest in pyramidal neurons in deep L3. Additionally, pharmacologically blocking h-channels produced a larger change in membrane properties in human compared to mouse neurons. Finally, using biophysical modeling, we provide evidence that h-channels promote the transfer of theta frequencies from dendrite-to-soma in human L3 pyramidal neurons. Thus, h-channels contribute to between-species differences in a fundamental neuronal property.
Keywords: Human; gene expression; h-channel; intrinsic membrane properties; mouse; neuron model; oscillations; patch-clamp physiology; pyramidal neuron.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests
Figures



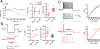

Similar articles
-
Homeostatic regulation of synaptic excitability: tonic GABA(A) receptor currents replace I(h) in cortical pyramidal neurons of HCN1 knock-out mice.J Neurosci. 2010 Feb 17;30(7):2611-22. doi: 10.1523/JNEUROSCI.3771-09.2010. J Neurosci. 2010. PMID: 20164346 Free PMC article.
-
HCN1 subunits contribute to the kinetics of I(h) in neonatal cortical plate neurons.Dev Neurobiol. 2013 Oct;73(10):785-97. doi: 10.1002/dneu.22104. Epub 2013 Aug 20. Dev Neurobiol. 2013. PMID: 23821600
-
Loss of HCN1 subunits causes absence epilepsy in rats.Brain Res. 2019 Mar 1;1706:209-217. doi: 10.1016/j.brainres.2018.11.004. Epub 2018 Nov 5. Brain Res. 2019. PMID: 30408474
-
Neurophysiology of HCN channels: from cellular functions to multiple regulations.Prog Neurobiol. 2014 Jan;112:1-23. doi: 10.1016/j.pneurobio.2013.10.001. Epub 2013 Oct 29. Prog Neurobiol. 2014. PMID: 24184323 Review.
-
Evolution of cortical neurons supporting human cognition.Trends Cogn Sci. 2022 Nov;26(11):909-922. doi: 10.1016/j.tics.2022.08.012. Epub 2022 Sep 15. Trends Cogn Sci. 2022. PMID: 36117080 Free PMC article. Review.
Cited by
-
Net synaptic drive of fast-spiking interneurons is inverted towards inhibition in human FCD I epilepsy.Nat Commun. 2024 Aug 6;15(1):6683. doi: 10.1038/s41467-024-51065-7. Nat Commun. 2024. PMID: 39107293 Free PMC article.
-
Translational Block in Stroke: A Constructive and "Out-of-the-Box" Reappraisal.Front Neurosci. 2021 May 14;15:652403. doi: 10.3389/fnins.2021.652403. eCollection 2021. Front Neurosci. 2021. PMID: 34054413 Free PMC article.
-
A Minimal Biophysical Model of Neocortical Pyramidal Cells: Implications for Frontal Cortex Microcircuitry and Field Potential Generation.J Neurosci. 2020 Oct 28;40(44):8513-8529. doi: 10.1523/JNEUROSCI.0221-20.2020. Epub 2020 Oct 9. J Neurosci. 2020. PMID: 33037076 Free PMC article.
-
Vulnerability of Human Cerebellar Neurons to Degeneration in Ataxia-Causing Channelopathies.Front Syst Neurosci. 2022 Jun 9;16:908569. doi: 10.3389/fnsys.2022.908569. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35757096 Free PMC article. Review.
-
Allometric rules for mammalian cortical layer 5 neuron biophysics.Nature. 2021 Dec;600(7888):274-278. doi: 10.1038/s41586-021-04072-3. Epub 2021 Nov 10. Nature. 2021. PMID: 34759318 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

