Prognostic effect of whole chromosomal aberration signatures in standard-risk, non-WNT/non-SHH medulloblastoma: a retrospective, molecular analysis of the HIT-SIOP PNET 4 trial
- PMID: 30392813
- PMCID: PMC6262170
- DOI: 10.1016/S1470-2045(18)30532-1
Prognostic effect of whole chromosomal aberration signatures in standard-risk, non-WNT/non-SHH medulloblastoma: a retrospective, molecular analysis of the HIT-SIOP PNET 4 trial
Abstract
Background: Most children with medulloblastoma fall within the standard-risk clinical disease group defined by absence of high-risk features (metastatic disease, large-cell/anaplastic histology, and MYC amplification), which includes 50-60% of patients and has a 5-year event-free survival of 75-85%. Within standard-risk medulloblastoma, patients in the WNT subgroup are established as having a favourable prognosis; however, outcome prediction for the remaining majority of patients is imprecise. We sought to identify novel prognostic biomarkers to enable improved risk-adapted therapies.
Methods: The HIT-SIOP PNET 4 trial recruited 338 patients aged 4-21 years with medulloblastoma between Jan 1, 2001, and Dec 31, 2006, in 120 treatment institutions in seven European countries to investigate hyperfractionated radiotherapy versus standard radiotherapy. In this retrospective analysis, we assessed the remaining tumour samples from patients in the HIT-SIOP PNET 4 trial (n=136). We assessed the clinical behaviour of the molecularly defined WNT and SHH subgroups, and identified novel independent prognostic markers and models for standard-risk patients with non-WNT/non-SHH disease. Because of the scarcity and low quality of available genomic material, we used a mass spectrometry-minimal methylation classifier assay (MS-MIMIC) to assess methylation subgroup and a molecular inversion probe array to detect genome-wide copy number aberrations. Prognostic biomarkers and models identified were validated in an independent, demographically matched cohort (n=70) of medulloblastoma patients with non-WNT/non-SHH standard-risk disease treated with conventional therapies (maximal surgical resection followed by adjuvant craniospinal irradiation [all patients] and chemotherapy [65 of 70 patients], at UK Children's Cancer and Leukaemia Group and European Society for Paediatric Oncology (SIOPE) associated treatment centres between 1990 and 2014. These samples were analysed by Illumina 450k DNA methylation microarray. HIT-SIOP PNET 4 is registered with ClinicalTrials.gov, number NCT01351870.
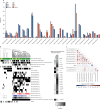
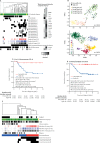
Findings: We analysed methylation subgroup, genome-wide copy number aberrations, and mutational features in 136 assessable tumour samples from the HIT-SIOP PNET 4 cohort, representing 40% of the 338 patients in the trial cohort. This cohort of 136 samples consisted of 28 (21%) classified as WNT, 17 (13%) as SHH, and 91 (67%) as non-WNT/non-SHH (we considered Group3 and Group4 medulloblastoma together in our analysis because of their similar molecular and clinical features). Favourable outcomes for WNT tumours were confirmed in patients younger than 16 years, and all relapse events in SHH (four [24%] of 17) occurred in patients with TP53 mutation (TP53mut) or chromosome 17p loss. A novel whole chromosomal aberration signature associated with increased ploidy and multiple non-random whole chromosomal aberrations was identified in 38 (42%) of the 91 samples from patients with non-WNT/non-SHH medulloblastoma in the HIT-SIOP PNET 4 cohort. Biomarkers associated with this whole chromosomal aberration signature (at least two of chromosome 7 gain, chromosome 8 loss, and chromosome 11 loss) predicted favourable prognosis. Patients with non-WNT/non-SHH medulloblastoma could be reclassified by these markers as having favourable-risk or high-risk disease. In patients in the HIT-SIOP PNET4 cohort with non-WNT/non-SHH medulloblastoma, with a median follow-up of 6·7 years (IQR 5·8-8·2), 5-year event-free survival was 100% in the favourable-risk group and 68% (95% CI 57·5-82·7; p=0·00014) in the high-risk group. In the validation cohort, with a median follow-up of 5·6 years (IQR 3·1-8·1), 5-year event-free survival was 94·7% (95% CI 85·2-100) in the favourable-risk group and 58·6% (95% CI 45·1-76·1) in the high-risk group (hazard ratio 9·41, 95% CI 1·25-70·57; p=0·029). Our comprehensive molecular investigation identified subgroup-specific risk models which allowed 69 (51%) of 134 accessible patients from the standard-risk medulloblastoma HIT-SIOP PNET 4 cohort to be assigned to a favourable-risk group.
Interpretation: We define a whole chromosomal signature that allows the assignment of non-WNT/non-SHH medulloblastoma patients normally classified as standard-risk into favourable-risk and high-risk categories. In addition to patients younger than 16 years with WNT tumours, patients with non-WNT/non-SHH tumours with our defined whole chromosomal aberration signature and patients with SHH-TP53wild-type tumours should be considered for therapy de-escalation in future biomarker-driven, risk-adapted clinical trials. The remaining subgroups of patients with high-risk medulloblastoma might benefit from more intensive therapies.
Funding: Cancer Research UK, Swedish Childhood Cancer Foundation, French Ministry of Health/French National Cancer Institute, and the German Children's Cancer Foundation.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures




Comment in
-
Interrogating molecular data for medulloblastoma risk stratification.Lancet Oncol. 2018 Dec;19(12):1548-1549. doi: 10.1016/S1470-2045(18)30585-0. Epub 2018 Nov 1. Lancet Oncol. 2018. PMID: 30392812 No abstract available.
Similar articles
-
Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort.Lancet Oncol. 2018 Jun;19(6):785-798. doi: 10.1016/S1470-2045(18)30242-0. Epub 2018 May 9. Lancet Oncol. 2018. PMID: 29753700 Free PMC article.
-
Risk-adapted therapy for young children with medulloblastoma (SJYC07): therapeutic and molecular outcomes from a multicentre, phase 2 trial.Lancet Oncol. 2018 Jun;19(6):768-784. doi: 10.1016/S1470-2045(18)30204-3. Epub 2018 May 16. Lancet Oncol. 2018. PMID: 29778738 Free PMC article. Clinical Trial.
-
Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study.Lancet Oncol. 2017 Jul;18(7):958-971. doi: 10.1016/S1470-2045(17)30243-7. Epub 2017 May 22. Lancet Oncol. 2017. PMID: 28545823 Free PMC article.
-
Risk stratification of childhood medulloblastoma in the molecular era: the current consensus.Acta Neuropathol. 2016 Jun;131(6):821-31. doi: 10.1007/s00401-016-1569-6. Epub 2016 Apr 4. Acta Neuropathol. 2016. PMID: 27040285 Free PMC article. Review.
-
The potential impact of tumour biology on improved clinical practice for medulloblastoma: progress towards biologically driven clinical trials.Br J Neurosurg. 2009 Aug;23(4):364-75. doi: 10.1080/02688690903121807. Br J Neurosurg. 2009. PMID: 19637007 Review.
Cited by
-
Biology and grading of pleomorphic xanthoastrocytoma-what have we learned about it?Brain Pathol. 2021 Jan;31(1):20-32. doi: 10.1111/bpa.12874. Epub 2020 Aug 4. Brain Pathol. 2021. PMID: 32619305 Free PMC article.
-
Treatment response as surrogate to predict risk for disease progression in pediatric medulloblastoma with persistent magnetic resonance imaging lesions after first-line treatment.Neuro Oncol. 2024 Sep 5;26(9):1712-1722. doi: 10.1093/neuonc/noae071. Neuro Oncol. 2024. PMID: 38578306 Free PMC article.
-
Advanced molecular pathology for rare tumours: A national feasibility study and model for centralised medulloblastoma diagnostics.Neuropathol Appl Neurobiol. 2021 Oct;47(6):736-747. doi: 10.1111/nan.12716. Epub 2021 May 2. Neuropathol Appl Neurobiol. 2021. PMID: 33826763 Free PMC article.
-
Molecular characterisation defines clinically-actionable heterogeneity within Group 4 medulloblastoma and improves disease risk-stratification.Acta Neuropathol. 2023 May;145(5):651-666. doi: 10.1007/s00401-023-02566-0. Epub 2023 Apr 4. Acta Neuropathol. 2023. PMID: 37014508 Free PMC article.
-
Risk factors for domain-specific neurocognitive outcome in pediatric survivors of a brain tumor in the posterior fossa-Results of the HIT 2000 trial.Neuro Oncol. 2024 Nov 4;26(11):2113-2124. doi: 10.1093/neuonc/noae092. Neuro Oncol. 2024. PMID: 38835160 Free PMC article.
References
-
- Ellison DW, Onilude OE, Lindsey JC. Beta-catenin status predicts a favourable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23:7951–7957. - PubMed
-
- Clifford SC, Lusher ME, Lindsey JC. Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle. 2006;5:2666–2670. - PubMed
-
- Pizer BL, Clifford SC. The potential impact of tumour biology on improved clinical practice for medulloblastoma: progress towards biologically driven clinical trials. Br J Neurosurg. 2009;23:364–375. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

