The GSK3β inhibitor, TDZD-8, rescues cognition in a zebrafish model of okadaic acid-induced Alzheimer's disease
- PMID: 30392874
- PMCID: PMC11956762
- DOI: 10.1016/j.neuint.2018.10.022
The GSK3β inhibitor, TDZD-8, rescues cognition in a zebrafish model of okadaic acid-induced Alzheimer's disease
Abstract
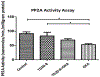
Currently, no treatments exist that are able to directly treat against Alzheimer's disease (AD), and we are facing an inevitable increase in the near future of the amount of patients who will suffer from AD. Most animal models of AD are limited by not being able to recapitulate the entire pathology of AD. Recently an AD model in zebrafish was established by using the protein phosphatase 2A inhibitor, okadaic acid (OKA). Administering OKA to zebrafish was able to recapitulate most of the neuropathology associated with AD. Therefore, providing a drug discovery model for AD that is also time and cost efficient. This study was designed to investigate the effects of GSK3β inhibition by 4-benzyl-2-methyl-1, 2, 4-thiadiazolidine-3, 5-dione (TDZD-8) on this newly developed AD model. Fish were divided into 4 groups and each group received a different treatment. The fish were divided into a control group, a group treated with 1 μM TDZD-8 only, a group treated with 1 μM TDZD-8 + 100 nM OKA, and a group treated with 100 nM OKA only. Administering the GSK3β inhibitor to zebrafish concomitantly with OKA proved to be protective. TDZD-8 treatment reduced the mortality rate, the ratio of active: inactive GSK3β, pTau (Ser199), and restored PP2A activity. This further corroborates the use of GSKβ inhibitors in the treatment against AD and bolsters the use of the OKA-induced AD-like zebrafish model for drug discovery.
Keywords: Alzheimer's disease; GSK3β; Okadaic acid; PP2A; TDZD-8; Tau; Zebrafish.
Copyright © 2018. Published by Elsevier Ltd.
Figures


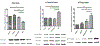
Similar articles
-
Okadaic acid-induced Tau phosphorylation in rat brain: role of NMDA receptor.Neuroscience. 2013 May 15;238:97-113. doi: 10.1016/j.neuroscience.2013.01.075. Epub 2013 Feb 13. Neuroscience. 2013. PMID: 23415789
-
The new indirubin derivative inhibitors of glycogen synthase kinase-3, 6-BIDECO and 6-BIMYEO, prevent tau phosphorylation and apoptosis induced by the inhibition of protein phosphatase-2A by okadaic acid in cultured neurons.J Neurosci Res. 2011 Nov;89(11):1802-11. doi: 10.1002/jnr.22723. Epub 2011 Aug 8. J Neurosci Res. 2011. PMID: 21826701
-
Tau phosphorylation and neuronal apoptosis induced by the blockade of PP2A preferentially involve GSK3β.Neurochem Int. 2011 Aug;59(2):235-50. doi: 10.1016/j.neuint.2011.05.010. Epub 2011 Jun 6. Neurochem Int. 2011. PMID: 21672577
-
Targeting of PP2 A/GSK3β/PTEN Axis in Alzheimer Disease: The Mooting Evidence, Divine, and Devil.Cell Mol Neurobiol. 2025 Apr 18;45(1):36. doi: 10.1007/s10571-025-01554-0. Cell Mol Neurobiol. 2025. PMID: 40251348 Free PMC article. Review.
-
Molecular and cellular mechanism of okadaic acid (OKA)-induced neurotoxicity: a novel tool for Alzheimer's disease therapeutic application.Mol Neurobiol. 2014 Dec;50(3):852-65. doi: 10.1007/s12035-014-8699-4. Epub 2014 Apr 8. Mol Neurobiol. 2014. PMID: 24710687 Review.
Cited by
-
Identification of Potential Kinase Inhibitors within the PI3K/AKT Pathway of Leishmania Species.Biomolecules. 2021 Jul 16;11(7):1037. doi: 10.3390/biom11071037. Biomolecules. 2021. PMID: 34356660 Free PMC article.
-
COVID-19: inflammatory responses, structure-based drug design and potential therapeutics.Mol Divers. 2022 Feb;26(1):629-645. doi: 10.1007/s11030-020-10176-1. Epub 2021 Jan 5. Mol Divers. 2022. PMID: 33400086 Free PMC article. Review.
-
GSK-3β Allosteric Inhibition: A Dead End or a New Pharmacological Frontier?Int J Mol Sci. 2023 Apr 19;24(8):7541. doi: 10.3390/ijms24087541. Int J Mol Sci. 2023. PMID: 37108703 Free PMC article. Review.
-
Sulfur-containing therapeutics in the treatment of Alzheimer's disease.Med Chem Res. 2021 Feb;30(2):305-352. doi: 10.1007/s00044-020-02687-1. Epub 2021 Jan 15. Med Chem Res. 2021. PMID: 33613018 Free PMC article.
-
Advances of Zebrafish in Neurodegenerative Disease: From Models to Drug Discovery.Front Pharmacol. 2021 Jul 14;12:713963. doi: 10.3389/fphar.2021.713963. eCollection 2021. Front Pharmacol. 2021. PMID: 34335276 Free PMC article. Review.
References
-
- Association A.s., 2018. Alzheimer’s disease facts and figures. Alzheimer’s Dementia 14 (3), 367–429 2018.
-
- Bhat RV, Budd Haeberlein SL, Avila J, 2004. Glycogen synthase kinase 3: a drug target for CNS therapies. J. Neurochem. 89 (6), 1313–1317. - PubMed
-
- Bradford MM, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

