T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation
- PMID: 30392957
- PMCID: PMC6239889
- DOI: 10.1016/j.cell.2018.10.008
T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation
Abstract
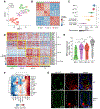
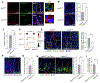
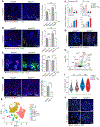
In the small intestine, a niche of accessory cell types supports the generation of mature epithelial cell types from intestinal stem cells (ISCs). It is unclear, however, if and how immune cells in the niche affect ISC fate or the balance between self-renewal and differentiation. Here, we use single-cell RNA sequencing (scRNA-seq) to identify MHC class II (MHCII) machinery enrichment in two subsets of Lgr5+ ISCs. We show that MHCII+ Lgr5+ ISCs are non-conventional antigen-presenting cells in co-cultures with CD4+ T helper (Th) cells. Stimulation of intestinal organoids with key Th cytokines affects Lgr5+ ISC renewal and differentiation in opposing ways: pro-inflammatory signals promote differentiation, while regulatory cells and cytokines reduce it. In vivo genetic perturbation of Th cells or MHCII expression on Lgr5+ ISCs impacts epithelial cell differentiation and IEC fate during infection. These interactions between Th cells and Lgr5+ ISCs, thus, orchestrate tissue-wide responses to external signals.
Keywords: ISCs; MHC class II; MHCII; T helper; T regulatory; T(reg); Th; epithelial differentiation; gut biology; intestinal stem cells; mucosal immunity; scRNA-seq; single cell RNA-seq; stem cell renewal; tuft cells.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures




Comment in
-
T cell help to stem cells.Nat Rev Immunol. 2018 Dec;18(12):730-731. doi: 10.1038/s41577-018-0089-0. Nat Rev Immunol. 2018. PMID: 30420703 No abstract available.
-
TH cells tune intestinal stem cell fate.Nat Rev Gastroenterol Hepatol. 2019 Jan;16(1):3. doi: 10.1038/s41575-018-0096-4. Nat Rev Gastroenterol Hepatol. 2019. PMID: 30523280 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS030843/NS/NINDS NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- K99 AG045144/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- R01 DK117263/DK/NIDDK NIH HHS/United States
- R00 AG045144/AG/NIA NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- U54 CA224068/CA/NCI NIH HHS/United States
- RM1 HG006193/HG/NHGRI NIH HHS/United States
- K99 EY028625/EY/NEI NIH HHS/United States
- RC2 DK114784/DK/NIDDK NIH HHS/United States
- U24 AI118672/AI/NIAID NIH HHS/United States
- R01 CA034992/CA/NCI NIH HHS/United States
- R01 CA211184/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

