Pulmonary and other health effects of electronic cigarette use among adult smokers participating in a randomized controlled smoking reduction trial
- PMID: 30393015
- PMCID: PMC6358505
- DOI: 10.1016/j.addbeh.2018.10.041
Pulmonary and other health effects of electronic cigarette use among adult smokers participating in a randomized controlled smoking reduction trial
Abstract
Background: There is limited evidence about the effects of dual electronic cigarette (e-cig) and combustible cigarette use on lung health or other health outcomes. Studies that have evaluated these outcomes have not included estimates of e-cig or cigarette exposure in the analyses.
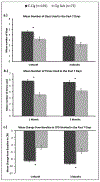
Materials and methods: Data analyzed were from 263 smokers participating in a randomized controlled trial designed to encourage participants to reduce their combustible cigarette use by substituting with an e-cig or a non-electronic cigarette substitute (cig-sub). t-tests were used to evaluate changes from baseline at 1 month and 3 months in lung function, blood pressure, pulse, exhaled carbon monoxide, and weight. Linear mixed effects models were used to test associations between health outcomes and study product group, including exposure to the study products (e-cig and cig-sub times used and days used in the past 7 days) and cigarettes per day (CPD).
Results: There were few significant differences between the groups for lung function indices at any time point in the unadjusted analyses. There were significant reductions in diastolic blood pressure and pulse at 1 month in the unadjusted analyses for those in the e-cig group compared to the cig-sub group. CPD decreased significantly more for the e-cig group than for the cig-sub group at both time points. There were no significant associations between any measured health outcomes and group in the linear mixed effects models.
Conclusion: E-cig use did not contribute to significant changes in health outcome markers as compared with use of a non-electronic cig-sub.
Keywords: Electronic cigarettes; Health effects; Lung function; Smoking reduction.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

