Multisensory Control of Orientation in Tethered Flying Drosophila
- PMID: 30393038
- PMCID: PMC6503675
- DOI: 10.1016/j.cub.2018.09.020
Multisensory Control of Orientation in Tethered Flying Drosophila
Abstract
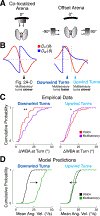
A longstanding goal of systems neuroscience is to quantitatively describe how the brain integrates sensory cues over time. Here, we develop a closed-loop orienting paradigm in Drosophila to study the algorithms by which cues from two modalities are integrated during ongoing behavior. We find that flies exhibit two behaviors when presented simultaneously with an attractive visual stripe and aversive wind cue. First, flies perform a turn sequence where they initially turn away from the wind but later turn back toward the stripe, suggesting dynamic sensory processing. Second, turns toward the stripe are slowed by the presence of competing wind, suggesting summation of turning drives. We develop a model in which signals from each modality are filtered in space and time to generate turn commands and then summed to produce ongoing orienting behavior. This computational framework correctly predicts behavioral dynamics for a range of stimulus intensities and spatial arrangements.
Keywords: Drosophila; behavior; integration; mechanosensation; multisensory; navigation; orientation; vision.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

