A Robust Transposon-Endogenizing Response from Germline Stem Cells
- PMID: 30393075
- PMCID: PMC6631374
- DOI: 10.1016/j.devcel.2018.10.011
A Robust Transposon-Endogenizing Response from Germline Stem Cells
Abstract
The heavy occupancy of transposons in the genome implies that existing organisms have survived from multiple, independent rounds of transposon invasions. However, how and which host cell types survive the initial wave of transposon invasion remain unclear. We show that the germline stem cells can initiate a robust adaptive response that rapidly endogenizes invading P element transposons by activating the DNA damage checkpoint and piRNA production. We find that temperature modulates the P element activity in germline stem cells, establishing a powerful tool to trigger transposon hyper-activation. Facing vigorous invasion, Drosophila first shut down oogenesis and induce selective apoptosis. Interestingly, a robust adaptive response occurs in ovarian stem cells through activation of the DNA damage checkpoint. Within 4 days, the hosts amplify P element-silencing piRNAs, repair DNA damage, subdue the transposon, and reinitiate oogenesis. We propose that this robust adaptive response can bestow upon organisms the ability to survive recurrent transposon invasions throughout evolution.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests
Figures
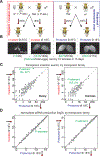

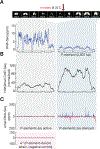
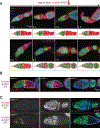



References
-
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, et al. (2006). A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207. - PubMed
-
- Aravin AA, Naumova NM, Tulin AV, Vagin VV, Rozovsky YM, and Gvozdev VA (2001). Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Current biology : CB 11, 1017–1027. - PubMed
-
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, and Hannon GJ (2007). Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128, 1089–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

