Prolactin-Induced Protein is a novel biomarker for Keratoconus
- PMID: 30393162
- PMCID: PMC6360109
- DOI: 10.1016/j.exer.2018.10.015
Prolactin-Induced Protein is a novel biomarker for Keratoconus
Abstract
Purpose: The purpose of the study was to investigate the role of Prolactin-Induced Protein (PIP) as a predictive biomarker for Keratoconus (KC).
Participants: This study included one hundred and forty-seven patients with KC (105 male, 42 female), and sixty healthy controls (27 male, 33 female).
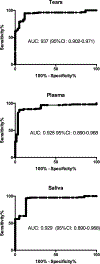
Methods: Tears, plasma and saliva samples were collected from all participants. In both KC and healthy groups all collected samples were divided into four age subgroups (15-24y), (25-34y), (35-44y) and (45y and up). Samples were analyzed using western blot (WB) and enzyme-linked immunosorbent assay (ELISA). Areas under the receiver operating characteristic curves (AUROCs) were used to evaluate diagnostic accuracy for distinguishing between KC and healthy eyes.
Main outcome measures: Difference in PIP protein levels between patients with KC and healthy controls.
Results: Results showed significant downregulation of PIP expression in all three biological fluids on KC patients when compared to healthy controls, independent of age, sex and severity. Since PIP is a hormonal-regulated protein, we also investigated the expression of major sex hormones. We detected significant upregulation in salivary and plasma Dehydroepiandrosterone sulfate (DHEA-S) levels and significant downregulation of estrone and estriol levels, in KC patients compared to healthy controls, independent of sex, age, and KC severity stage. ROC was used to determine the overall predictive accuracy of this protein in KC. Data showed an area under the curve (AUC) for PIP in tears of 0.937 (95%CI: 0.902-0.971), in plasma of 0.928 (95%CI: 0.890-0.968) and in saliva of 0.929 (95%CI: 0.890-0.968).
Conclusions: Conclusively, our results show that PIP levels are reduced in all three human biological fluids tested, and may independently or in combination with current imaging techniques aid in screening and diagnosis of KC. Our data revealed that PIP levels can potentially differentiate between disease and healthy cases, and PIP levels are stable in relation to KC severity, sex and age. Moreover, alterations in sex hormone levels in correlation with reduced PIP levels in KC provide an intriguing insight in the underlying KC pathophysiology and highlights the role of PIP as a KC biomarker.
Keywords: Biomarker; Blood; Keratoconus; Prolactin-Induced Protein; Saliva; Tears.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures



References
-
- Baldini C, Ferro F, Elefante E, Bombardieri S, 2018. Biomarkers for Sjögren’s syndrome. Biomarkers in Medicine 12, 275–286. - PubMed
-
- Carsol JL, Gingras S, Simard J, 2002. Synergistic action of prolactin (PRL) and androgen on PRL-inducible protein gene expression in human breast cancer cells: a unique model for functional cooperation between signal transducer and activator of transcription-5 and androgen receptor. Mol Endocrinol 16, 1696–1710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

