MicroRNA-32 Regulates Development and Progression of Hepatocellular Carcinoma by Targeting ADAMTS9 and Affects Its Prognosis
- PMID: 30393368
- PMCID: PMC6237041
- DOI: 10.12659/MSMBR.910522
MicroRNA-32 Regulates Development and Progression of Hepatocellular Carcinoma by Targeting ADAMTS9 and Affects Its Prognosis
Abstract
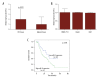
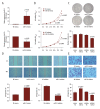
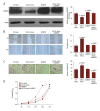
BACKGROUND MicroRNA-32 (miR-32) induces cell proliferation and metastasis in hepatocellular carcinoma (HCC), but the detailed mechanisms of miR-32 in regulating oncogenesis and development of HCC have not been clarified. The aim of this study was to investigate the effects of miR-32 on HCC and its clinical pathological significance, as well as to determine the functional connection between miR-32 and ADAMTS9 in HCC. MATERIAL AND METHODS Quantitative RT-PCR was used to assess the expression levels of miR-32 in HCC tissues, adjacent non-cancerous tissues, and liver cancer cell lines. In vitro cell proliferation, migration, and invasion assays were performed to confirm the biological functions of miR-32. Quantitative RT-PCR, Western blot analysis, and luciferase reporter assays were used to evaluate the role of miR-32 in the regulation of ADAMTS9. RESULTS miR-32 was highly expressed in HCC tissues compared with corresponding adjacent non-cancerous tissues. Over-expression of miR-32 was also found in 3 human liver cancer cell lines: SMMC-7721, Huh7, and HepG2. Moreover, increasing expression of miR-32 in HCC tissues was related to shorter overall survival. In vitro over-expression of miR-32 promoted cell proliferation, migration, and invasion; however, the under-expression of miR-32 revealed the opposite effects. Dual-luciferase reporter assay indicated that miR-32 can directly bind to the 3'-UTR of ADAMTS9. Western blot analysis showed that over-expression of miR-32 decreased expression of ADAMTS9 protein. Rescue tests further verified the connection between miR-32 and ADAMTS9. CONCLUSIONS Our data indicate that miR-32 accelerates progression in HCC by targeting ADAMTS9, and the abnormal expression of miR-32 is correlated with prognosis and could become a potential therapeutic target.
Conflict of interest statement
None.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

