The functional significance of dentin sialoprotein-phosphophoryn and dentin sialoprotein
- PMID: 30393383
- PMCID: PMC6215839
- DOI: 10.1038/s41368-018-0035-9
The functional significance of dentin sialoprotein-phosphophoryn and dentin sialoprotein
Abstract
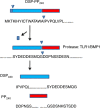
Phosphophoryn (PP) and dentin sialoprotein (DSP) are the most dominant non-collagenous proteins in dentin. PP is an extremely acidic protein that can function as a mineral nucleator for dentin mineralization. DSP was first identified in 1981, yet its functional significance is still controversial. Historically, these two proteins were considered to be independently synthesized and secreted by dental pulp cells into the developing dentin matrix. However, with the identification of the DSP coding sequence in 1994, followed 2 years later by the finding that the PP coding sequence was located immediately downstream from the DSP sequence, it became immediately clear that DSP and PP proteins were derived from a single DSP-PP (i.e., dentin sialophosphoprotein, DSPP) transcript. Since DSPP cDNA became available, tremendous progress has been made in studying DSP-PP mRNA distribution and DSP generation from the DSP-PP precursor protein at specific cleavage sites by protease tolloid-related-1 (TLR1) or bone morphogenetic protein 1 (BMP1). The functions of DSP-PP and DSP were investigated via DSP-PP knockout (KO) and DSP knockin in DSP-PP KO mice. In addition, a number of in vitro studies aimed to elucidate DSPP and DSP function in dental pulp cells.
Conflict of interest statement
There is no conflict of interests.
Figures

 ;
;  Represents DSP exons;
Represents DSP exons;  Represents PP exon;
Represents PP exon;  Represents the 3′ noncoding sequence;
Represents the 3′ noncoding sequence;  Represents the DSP-PP promoter region;
Represents the DSP-PP promoter region;  Represents the transcription start site;
Represents the transcription start site;  Represents the stop codon
Represents the stop codon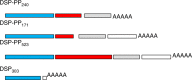

References
-
- Linde A, Bhown M, Butler WT. Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J. Biol. Chem. 1980;255:5931–5942. - PubMed
-
- Veis, A. in Chemistry and Biology of Mineralized Tissues (ed. Butler, W. T.) 170–184 (EBSCO Media, Birmingham, 1985).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

