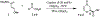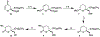Asymmetric hetero Diels-Alder route to quaternary carbon centers: synthesis of (-)-malyngolide
- PMID: 30393409
- PMCID: PMC6214206
- DOI: 10.1016/S0040-4039(01)01227-8
Asymmetric hetero Diels-Alder route to quaternary carbon centers: synthesis of (-)-malyngolide
Abstract
Conformationally constrained chiral bis(oxazoline)-metal complex catalyzed asymmetric hetero Diels-Alder reactions of Danishefsky's diene and a variety of α-keto esters constructed quaternary carbon centers enantioselectively. The reaction was utilized in the synthesis of (-)-malyngolide.
Figures
Similar articles
-
ASYMMETRIC HETERO DIELS-ALDER REACTIONS OF DANISHEFSKY'S DIENE AND GLYOXYLATE ESTERS CATALYZED BY CHIRAL BISOXAZOLINE DERIVED CATALYSTS.Tetrahedron Asymmetry. 1996 Aug;7(8):2165-2168. doi: 10.1016/0957-4166(96)00261-3. Tetrahedron Asymmetry. 1996. PMID: 30395656 Free PMC article.
-
A highly enantioselective hetero-Diels-Alder reaction of aldehydes with Danishefsky's diene catalyzed by chiral titanium(IV) 5,5',6,6',7,7',8,8'-octahydro-1,1'-bi-2-naphthol complexes.J Org Chem. 2002 Apr 5;67(7):2175-82. doi: 10.1021/jo016240u. J Org Chem. 2002. PMID: 11925225
-
Asymmetric hetero-Diels-Alder reaction of Danishefsky's diene with α-ketoesters and isatins catalyzed by a chiral N,N'-dioxide/magnesium(II) complex.Chemistry. 2014 Oct 27;20(44):14493-8. doi: 10.1002/chem.201404144. Epub 2014 Sep 11. Chemistry. 2014. PMID: 25213382
-
The asymmetric hetero-Diels-Alder reaction in the syntheses of biologically relevant compounds.Angew Chem Int Ed Engl. 2014 Oct 13;53(42):11146-57. doi: 10.1002/anie.201404094. Epub 2014 Sep 12. Angew Chem Int Ed Engl. 2014. PMID: 25220929 Review.
-
Recent Advances in Inverse-Electron-Demand Hetero-Diels-Alder Reactions of 1-Oxa-1,3-Butadienes.Top Curr Chem (Cham). 2016 Jun;374(3):24. doi: 10.1007/s41061-016-0026-2. Epub 2016 Apr 20. Top Curr Chem (Cham). 2016. PMID: 27573264 Free PMC article. Review.
Cited by
-
Capturing the essence of organic synthesis: from bioactive natural products to designed molecules in today's medicine.J Org Chem. 2010 Dec 3;75(23):7967-89. doi: 10.1021/jo101606g. Epub 2010 Oct 11. J Org Chem. 2010. PMID: 20936876 Free PMC article.
-
Investigation of the Anti-Methicillin-Resistant Staphylococcus aureus Activity of (+)-Tanikolide- and (+)-Malyngolide-Based Analogues Prepared by Asymmetric Synthesis.Int J Mol Sci. 2021 Jun 15;22(12):6400. doi: 10.3390/ijms22126400. Int J Mol Sci. 2021. PMID: 34203787 Free PMC article.
References
-
- Terada M; Mikami K; Nakai T Tetrahedron Lett. 1991, 32, 935;
- Corey EJ; Cywin CL; Roper TD Tetrahedron Lett. 1992, 33, 6907;
- Gao Q; Maruyama T; Mouri M; Yamamoto HJ Org. Chem 1992,57, 1957;
- Mikami K; Motoyama Y; Terada MJ Am. Chem. Soc 1994, 116, 2811;
- Motoyama Y; Mikami K J. Chem. Soc., Chem. Commun 1994, 1563;
- Keck GE; Li X-Y; Krishnamurthy D J.Org. Chem 1995, 60, 5998.
-
-
For excellent recent reviews, see:
Johnson JS; Evans DA Acc. Chem. Res 2000, 33, 325;Sibi MP; Porter NA Acc. Chem. Res 1999, 32, 163.
-
-
- Johannsen M; Yao S; Jørgensen KA Chem. Commun 1997, 2169;
- Yao S; Johannsen M; Audrain H; Hazell RG; Jorgensen KA J. Am. Chem. Soc 1998, 120, 8599.
-
-
For excellent reviews on quaternary carbon centers with four carbon substituents, see:
Corey EJ; Guzman-Perez A Angew. Chem., Int. Ed. Engl 1996, 37, 388;Fuji, K. Chem. Rev 1993, 93, 2037.and references cited therein.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous