Development of bioresources in Okinawa: understanding the multiple targeted actions of antioxidant phytochemicals
- PMID: 30393428
- PMCID: PMC6206290
- DOI: 10.1293/tox.2018-0041
Development of bioresources in Okinawa: understanding the multiple targeted actions of antioxidant phytochemicals
Abstract
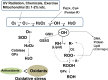
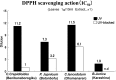
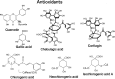
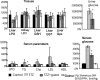
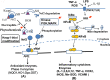
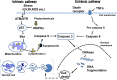
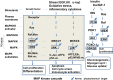
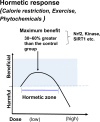
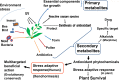
In research to develop healthy foods or preventive medicines from edible and medicinal herbs in Okinawa, we focused on the antioxidant activities of those bioresources. We first confirmed that the herbal antioxidant activities of such herbs increased upon ultraviolet irradiation treatment. This observation explains the high antioxidant activity of Okinawan vegetables, which grow under exposure to stronger ultraviolet light compared with those in other prefectures in Japan. Antidiabetic, hepatoprotective, cancer preventive, and cardioprotective actions were clarified using herbal extracts, and quercetin, chlorogenic acid, and gallic acid derivatives were isolated as antioxidant components from the herbs. Dimerumic acid was also isolated from the mold Monascus anka. All these antioxidants showed strong radical scavenging activities in vitro and beneficial effects in animal models. However, the concentrations of these compounds used in vivo seemed to be too low to have a physiologically important antioxidant effect based on their radical scavenging activities in vitro. Therefore, I performed a literature survey of antioxidant activities in vivo. Accumulating evidence has emerged that antioxidant phytochemicals show not only radical scavenging activities in vitro but also pleiotropic actions in vivo. The multitargeted, beneficial effects of antioxidant phytochemicals can be rationally explained using the xenohormesis concept, in which phytochemicals are the products of plant evolutionary adaptation to stress in plants, and their ability to induce a stress-adaptive response has been evolutionarily conserved in animals.
Keywords: antioxidant; multitargeted action; phytochemicals; stress adaptive response; xenohormesis.
Figures

Similar articles
-
Dimerumic acid as an antioxidant of the mold, Monascus anka.Free Radic Biol Med. 2000 Mar 15;28(6):999-1004. doi: 10.1016/s0891-5849(00)00188-x. Free Radic Biol Med. 2000. PMID: 10802232
-
Cardioprotective effects of extracts from Psidium guajava L and Limonium wrightii, Okinawan medicinal plants, against ischemia-reperfusion injury in perfused rat hearts.Pharmacology. 2003 Mar;67(3):128-35. doi: 10.1159/000067799. Pharmacology. 2003. PMID: 12571408
-
Dimerumic acid as an antioxidant from the mold, Monascus anka: the inhibition mechanisms against lipid peroxidation and hemeprotein-mediated oxidation.Biochem Pharmacol. 2002 Mar 1;63(5):1019-26. doi: 10.1016/s0006-2952(01)00923-6. Biochem Pharmacol. 2002. PMID: 11911855
-
Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases.Molecules. 2015 Nov 27;20(12):21138-56. doi: 10.3390/molecules201219753. Molecules. 2015. PMID: 26633317 Free PMC article. Review.
-
The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load.J Am Coll Nutr. 2009 Aug;28 Suppl:500S-516S. doi: 10.1080/07315724.2009.10718117. J Am Coll Nutr. 2009. PMID: 20234038 Review.
Cited by
-
Ingestion of Okinawa Island Vegetables Increases IgA Levels and Prevents the Spread of Influenza RNA Viruses.Nutrients. 2021 May 22;13(6):1773. doi: 10.3390/nu13061773. Nutrients. 2021. PMID: 34067427 Free PMC article.
References
-
- Halliwell B, and Gutteridge JMC. Free Radicals in Biology and Medicine 4th ed. Oxford University Press. Oxford. 2007.
Publication types
LinkOut - more resources
Full Text Sources
