Wiskott-Aldrich syndrome gene mutations modulate cancer susceptibility in the p53± murine model
- PMID: 30393584
- PMCID: PMC6209425
- DOI: 10.1080/2162402X.2018.1468954
Wiskott-Aldrich syndrome gene mutations modulate cancer susceptibility in the p53± murine model
Abstract
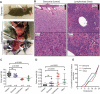
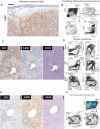
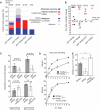
The Wiskott-Aldrich syndrome protein (WASp) is a key regulator of the actin cytoskeleton in hematopoietic cells and mutated in two severe immunodeficiency diseases with high incidence of cancer. Wiskott-Aldrich syndrome (WAS) is caused by loss-of-function mutations in WASp and most frequently associated with lymphoreticular tumors of poor prognosis. X-linked neuropenia (XLN) is caused by gain-of-function mutations in WASp and associated with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). To understand the role of WASp in tumorigenesis, we bred WASp+, WASp-, and WASp-XLN mice onto tumor susceptible p53+/- background and sub-lethally irradiated them to enhance tumor development. We followed the cohorts for 24 weeks and tumors were characterized by histology and flow cytometry to define the tumor incidence, onset, and cell origin. We found that p53+/-WASp+ mice developed malignancies, including solid tumors and T cell lymphomas with 71.4% of survival 24 weeks after irradiation. p53+/-WASp- mice showed lower survival rate and developed various early onset malignancies. Surprisingly, the p53+/-WASp-XLN mice developed malignancy mostly with late onset, which caused delayed mortality in this colony. This study provides evidence for that loss-of-function and gain-of-function mutations in WASp influence tumor incidence and onset.
Keywords: WASp; genetic model; immunodeficiency; malignancies; p53.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
