Metal-Organic Framework-Based Sensors for Environmental Contaminant Sensing
- PMID: 30393712
- PMCID: PMC6199112
- DOI: 10.1007/s40820-018-0218-0
Metal-Organic Framework-Based Sensors for Environmental Contaminant Sensing
Abstract
Increasing demand for timely and accurate environmental pollution monitoring and control requires new sensing techniques with outstanding performance, i.e., high sensitivity, high selectivity, and reliability. Metal-organic frameworks (MOFs), also known as porous coordination polymers, are a fascinating class of highly ordered crystalline coordination polymers formed by the coordination of metal ions/clusters and organic bridging linkers/ligands. Owing to their unique structures and properties, i.e., high surface area, tailorable pore size, high density of active sites, and high catalytic activity, various MOF-based sensing platforms have been reported for environmental contaminant detection including anions, heavy metal ions, organic compounds, and gases. In this review, recent progress in MOF-based environmental sensors is introduced with a focus on optical, electrochemical, and field-effect transistor sensors. The sensors have shown unique and promising performance in water and gas contaminant sensing. Moreover, by incorporation with other functional materials, MOF-based composites can greatly improve the sensor performance. The current limitations and future directions of MOF-based sensors are also discussed.
Keywords: Electrochemical sensor; Environmental contaminant; Field-effect transistor sensor; Metal–organic frameworks; Micro- and nanostructure; Optical sensor.
Figures



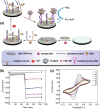
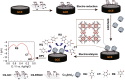
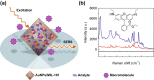

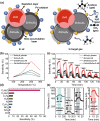

References
-
- Petrovic M, Farre M, de Alda ML, Perez S, Postigo C, Kock M, Radjenovic J, Gros M, Barcelo D. Recent trends in the liquid chromatography–mass spectrometry analysis of organic contaminants in environmental samples. J. Chromatogr. A. 2010;1217(25):4004–4017. doi: 10.1016/j.chroma.2010.02.059. - DOI - PubMed
-
- Falcaro P, Ricco R, Yazdi A, Imaz I, Furukawa S, Maspoch D, Ameloot R, Evans JD, Doonan CJ. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016;307:237–254. doi: 10.1016/j.ccr.2015.08.002. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
