Universal cancer screening: revolutionary, rational, and realizable
- PMID: 30393772
- PMCID: PMC6206005
- DOI: 10.1038/s41698-018-0066-x
Universal cancer screening: revolutionary, rational, and realizable
Abstract
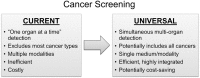
Cancer remains the second leading cause of mortality worldwide, and overall cancer-related deaths are increasing. Despite the survival benefit from early detection, screening has to date targeted only those few organs that harbor tumors of sufficient prevalence to show cost-effectiveness at population levels, leaving most cancer types unscreened. In this perspective overview, a case is made for universal cancer screening as a logical and more inclusive approach with potentially high impact. The centrally important conceptual drivers to universal screening are biological and epidemiological. The shared biology of tumor marker release into a common distant medium, like blood, can be exploited for multi-cancer detection from a single test. And, by aggregating prevalence rates, universal screening allows all cancers (including less common ones) to be included as targets, increases screening efficiency and integration across tumor types, and potentially improves cost-effectiveness over single-organ approaches. The identification of new tumor marker classes with both broad expression across tumor types and site-prediction, remarkable advances in assay technologies, and compelling early clinical data increase the likelihood of actualizing this new paradigm. Multi-organ screening could be achieved by targeting markers within or stemming from the circulation (including blood, urine, saliva, and expired breath) or those exfoliated into common excretory pathways (including the gastrointestinal and female reproductive tracts). Rigorous clinical studies in intended use populations and collaborations between academia, industry, professional societies, and government will be required to bring this lofty vision to a population application.
Conflict of interest statement
D.A.A. is co-inventor of the multi-target stool DNA test (Cologuard) and receives a portion of royalties from Exact Sciences to Mayo Clinic in accordance with Mayo Clinic policy. Mayo Clinic has an umbrella agreement with Exact Sciences to co-develop next generation molecular diagnostics for cancer detection; D.A.A. and his research team have contributed substantial intellectual property in this effort that has been licensed to Exact Sciences. The views expressed in this article are those of D.A.A. and do not reflect inputs from either Mayo Clinic or Exact Sciences.
Figures

References
-
- Kim J, Lee SK, Kim S, Koo MY, Choi M-Y, Cho DH, Bae SY, Lee J, Jung SP, Lee JE, Yang J-H, Nam SJ. P5-14-02: Clinicopathologic and Prognostic Difference of Screen Detected Breast Cancer Compared with Symptomatic Breast Cancer. Cancer Research. 2011;71(24 Supplement):P5-14-02-P5-14-02.
Publication types
LinkOut - more resources
Full Text Sources
Medical

