Functional Voltage-Gated Calcium Channels Are Present in Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium
- PMID: 30394009
- PMCID: PMC6344904
- DOI: 10.1002/sctm.18-0026
Functional Voltage-Gated Calcium Channels Are Present in Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium
Abstract
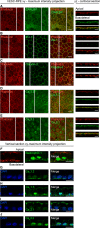
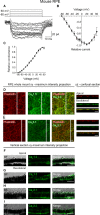
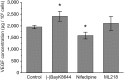
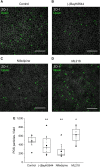
Retinal pigment epithelium (RPE) performs important functions for the maintenance of photoreceptors and vision. Malfunctions within the RPE are implicated in several retinal diseases for which transplantations of stem cell-derived RPE are promising treatment options. Their success, however, is largely dependent on the functionality of the transplanted cells. This requires correct cellular physiology, which is highly influenced by the various ion channels of RPE, including voltage-gated Ca2+ (CaV ) channels. This study investigated the localization and functionality of CaV channels in human embryonic stem cell (hESC)-derived RPE. Whole-cell patch-clamp recordings from these cells revealed slowly inactivating L-type currents comparable to freshly isolated mouse RPE. Some hESC-RPE cells also carried fast transient T-type resembling currents. These findings were confirmed by immunostainings from both hESC- and mouse RPE that showed the presence of the L-type Ca2+ channels CaV 1.2 and CaV 1.3 as well as the T-type Ca2+ channels CaV 3.1 and CaV 3.2. The localization of the major subtype, CaV 1.3, changed during hESC-RPE maturation co-localizing with pericentrin to the base of the primary cilium before reaching more homogeneous membrane localization comparable to mouse RPE. Based on functional assessment, the L-type Ca2+ channels participated in the regulation of vascular endothelial growth factor secretion as well as in the phagocytosis of photoreceptor outer segments in hESC-RPE. Overall, this study demonstrates that a functional machinery of voltage-gated Ca2+ channels is present in mature hESC-RPE, which is promising for the success of transplantation therapies. Stem Cells Translational Medicine 2019;8:179&15.
Keywords: Patch-clamp; Phagocytosis; Retinal pigment epithelium; Stem cells; Vascular endothelial growth factor; Voltage-gated Ca2+ channels.
© 2018 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures



Similar articles
-
Heterogeneity of Potassium Channels in Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium.Stem Cells Transl Med. 2022 Jul 20;11(7):753-766. doi: 10.1093/stcltm/szac029. Stem Cells Transl Med. 2022. PMID: 35639962 Free PMC article.
-
Sodium channels enable fast electrical signaling and regulate phagocytosis in the retinal pigment epithelium.BMC Biol. 2019 Aug 15;17(1):63. doi: 10.1186/s12915-019-0681-1. BMC Biol. 2019. PMID: 31412898 Free PMC article.
-
Novel roles for voltage-gated T-type Ca2+ and ClC-2 channels in phagocytosis and angiogenic factor balance identified in human iPSC-derived RPE.FASEB J. 2021 Apr;35(4):e21406. doi: 10.1096/fj.202002754R. FASEB J. 2021. PMID: 33724552
-
Ca2+-channels in the RPE.Adv Exp Med Biol. 2002;514:225-35. Adv Exp Med Biol. 2002. PMID: 12596924 Review.
-
Ion channels and transporters of the retinal pigment epithelium.Exp Eye Res. 2014 Sep;126:27-37. doi: 10.1016/j.exer.2014.05.005. Exp Eye Res. 2014. PMID: 25152360 Review.
Cited by
-
Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells (MA09-hRPE) in macular degeneration.NPJ Regen Med. 2019 Aug 27;4:19. doi: 10.1038/s41536-019-0081-8. eCollection 2019. NPJ Regen Med. 2019. PMID: 31482011 Free PMC article. Review.
-
Culture surface protein coatings affect the barrier properties and calcium signalling of hESC-RPE.Sci Rep. 2021 Jan 13;11(1):933. doi: 10.1038/s41598-020-79638-8. Sci Rep. 2021. PMID: 33441679 Free PMC article.
-
Rev-Erbα and Photoreceptor Outer Segments modulate the Circadian Clock in Retinal Pigment Epithelial Cells.Sci Rep. 2019 Aug 13;9(1):11790. doi: 10.1038/s41598-019-48203-3. Sci Rep. 2019. PMID: 31409842 Free PMC article.
-
Ion channels research in hPSC-RPE cells: bridging benchwork to clinical applications.J Transl Med. 2024 Nov 27;22(1):1073. doi: 10.1186/s12967-024-05769-5. J Transl Med. 2024. PMID: 39604931 Free PMC article. Review.
-
Parkinsonism Sac domain mutation in Synaptojanin-1 affects ciliary properties in iPSC-derived dopaminergic neurons.Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2318943121. doi: 10.1073/pnas.2318943121. Epub 2024 Apr 18. Proc Natl Acad Sci U S A. 2024. PMID: 38635628 Free PMC article.
References
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev 2005;85:845‐881. - PubMed
-
- Wimmers S, Karl MO, Strauss O. Ion channels in the RPE. Prog Retin Eye Res 2007;26:263‐301. - PubMed
-
- Wong WL, Su X, Li X et al. Global prevalence of age‐related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta‐analysis. Lancet Glob Health 2014;2:e106‐e116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

