Subcellular compartmentalization of glutathione peroxidase 1 allelic isoforms differentially impact parameters of energy metabolism
- PMID: 30394058
- PMCID: PMC6336513
- DOI: 10.1002/jcb.27610
Subcellular compartmentalization of glutathione peroxidase 1 allelic isoforms differentially impact parameters of energy metabolism
Abstract
Specific genetic variations in the gene for the selenium-containing antioxidant protein glutathione peroxidase 1 (GPX1) are associated with the risk of a variety of common diseases, including cancer, diabetes, and cardiovascular disorders. Two common variations have been focused upon, one resulting in leucine or proline at codon 198 and another resulting in 5, 6, or 7 alanine repeats were previously shown to affect the distribution of GPX1 between the cytoplasm and mitochondria. Human MCF7 cells engineered to exclusively express GPX1 with five alanine repeats at amino terminus and proline at codon 198 (A5P) and seven alanine repeats at amino terminus and leucine at codon 198 (A7L), as well as derivatives targeted to the mitochondria by the addition of a mitochondrial localization sequence (mA5P and mA7L) were used to assess the consequences of the expression of these proteins on the cellular redox state and bioenergetics. Ectopic expression of A5P and A7L reduced the levels of reactive oxygen species, and the mitochondrially targeted derivatives exhibited better activity in these assays. Bioenergetics and mitochondrial integrity were assessed by measuring mitochondrial membrane potential, oxygen consumption, adenosine triphosphate (ATP) levels, and the levels of lactate dehydrogenase. The results of these assays indicated distinctively, and sometimes opposing, patterns with regard to differences between the consequences of the expression of A5P, A7L, mA5P, and mA7L. These data provide new information on the consequences of differences in the primary structure and cellular location of GPX1 proteins and contribute to the understanding of how these effects might contribute to human disease.
Keywords: gene polymorphisms; glutathione peroxidase (GPX); mitochondria; oxidative stress; respiration.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures




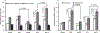

References
-
- Brigelius-Flohe R, Maiorino M. 2013. Glutathione peroxidaseseditor^editors. Biochim Biophys Acta. p 3289–303. - PubMed
-
- Ekoue DN, Bera S, Ansong E, Hart PC, Zaichick S, Domann FE, Bonini MG, Diamond AM. 2017. Allele-specific interaction between glutathione peroxidase 1 and manganese superoxide dismutase affects the levels of Bcl-2, Sirt3 and E-cadherin. Free Radic Res 51:582–590. 10.1080/10715762.2017.1339303. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

