Invited Review: APOE at the interface of inflammation, neurodegeneration and pathological protein spread in Alzheimer's disease
- PMID: 30394574
- PMCID: PMC6563457
- DOI: 10.1111/nan.12529
Invited Review: APOE at the interface of inflammation, neurodegeneration and pathological protein spread in Alzheimer's disease
Abstract
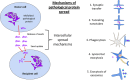
Despite more than a century of research, the aetiology of sporadic Alzheimer's disease (AD) remains unclear and finding disease modifying treatments for AD presents one of the biggest medical challenges of our time. AD pathology is characterized by deposits of aggregated amyloid beta (Aβ) in amyloid plaques and aggregated tau in neurofibrillary tangles. These aggregates begin in distinct brain regions and spread throughout the brain in stereotypical patterns. Neurodegeneration, comprising loss of synapses and neurons, occurs in brain regions with high tangle pathology, and an inflammatory response of glial cells appears in brain regions with pathological aggregates. Inheriting an apolipoprotein E ε4 (APOE4) allele strongly increases the risk of developing AD for reasons that are not yet entirely clear. Substantial amounts of evidence support a role for APOE in modulating the aggregation and clearance of Aβ, and data have been accumulating recently implicating APOE4 in exacerbating neurodegeneration, tau pathology and inflammation. We hypothesize that APOE4 influences all the pathological hallmarks of AD and may sit at the interface between neurodegeneration, inflammation and the spread of pathologies through the brain. Here, we conducted a systematic search of the literature and review evidence supporting a role for APOE4 in neurodegeneration and inflammation. While there is no direct evidence yet for APOE4 influencing the spread of pathology, we postulate that this may be found in future based on the literature reviewed here. In conclusion, this review highlights the importance of understanding the role of APOE in multiple important pathological mechanisms in AD.
Keywords: APOE; Alzheimer's disease; Apolipoprotein E; glia; inflammation; neurodegeneration; tau.
© 2018 The Authors. Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Figures




References
-
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993; 261: 921–3 - PubMed
-
- Li YJ, Hauser MA, Scott WK, Martin ER, Booze MW, Qin XJ, et al Apolipoprotein E controls the risk and age at onset of Parkinson disease. Neurology 2004; 62: 2005–9 - PubMed
-
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, et al Apolipoprotein e gene variability and cognitive functions at age 79: a follow‐up of the Scottish mental survey of 1932. Psychol Aging 2004; 19: 367–71 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

