Allenoates in Enantioselective [2+2] Cycloadditions: From a Mechanistic Curiosity to a Stereospecific Transformation
- PMID: 30394735
- PMCID: PMC6295144
- DOI: 10.1021/jacs.8b10008
Allenoates in Enantioselective [2+2] Cycloadditions: From a Mechanistic Curiosity to a Stereospecific Transformation
Abstract
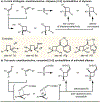
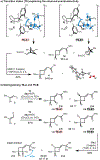
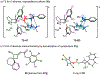
Identification of a novel catalyst-allenoate pair allows enantioselective [2+2] cycloaddition of α-methylstyrene. To understand the origin of selectivity, a detailed mechanistic investigation was conducted. Herein, two competing reaction pathways are proposed, which operate simultaneously and funnel the alkenes to the same axially chiral cyclobutanes. In agreement with the Woodward-Hoffmann rules, this mechanistic curiosity can be rationalized through a unique symmetry operation that was elucidated by deuteration experiments. In the case of 1,1-diarylalkenes, distal communication between the catalyst and alkene is achieved through subtle alteration of electronic properties and conformation. In this context, a Hammett study lends further credibility to a concerted mechanism. Thus, extended scope exploration, including β-substitution on the alkene to generate two adjacent stereocenters within the cyclobutane ring, is achieved in a highly stereospecific and enantioselective fashion (33 examples, up to >99:1 er).
Conflict of interest statement
The authors declare no competing financial interest.
Figures

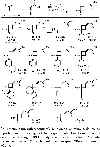


Similar articles
-
Lewis Acid-Promoted [2 + 2] Cycloadditions of Allenes and Ketenes: Versatile Methods for Natural Product Synthesis.Acc Chem Res. 2023 Aug 15;56(16):2253-2264. doi: 10.1021/acs.accounts.3c00334. Epub 2023 Aug 4. Acc Chem Res. 2023. PMID: 37540783 Free PMC article.
-
Synthesis of (-)-Hebelophyllene E: An Entry to Geminal Dimethyl-Cyclobutanes by [2+2] Cycloaddition of Alkenes and Allenoates.Angew Chem Int Ed Engl. 2018 Apr 16;57(17):4647-4651. doi: 10.1002/anie.201801110. Epub 2018 Mar 24. Angew Chem Int Ed Engl. 2018. PMID: 29481716 Free PMC article.
-
Concerted Enantioselective [2+2] Cycloaddition Reaction of Imines Mediated by a Magnesium Catalyst.J Am Chem Soc. 2023 Jan 11;145(1):610-625. doi: 10.1021/jacs.2c11284. Epub 2022 Dec 20. J Am Chem Soc. 2023. PMID: 36538490
-
Woodward-Hoffmann or Hoffmann-Woodward? Cycloadditions and the Transformation of Roald Hoffmann from a "Calculator" to an "Explainer".Chem Rec. 2024 Aug;24(8):e202300181. doi: 10.1002/tcr.202300181. Chem Rec. 2024. PMID: 39188247 Review.
-
Chiral Diol-Based Organocatalysts in Enantioselective Reactions.Molecules. 2018 Sep 11;23(9):2317. doi: 10.3390/molecules23092317. Molecules. 2018. PMID: 30208621 Free PMC article. Review.
Cited by
-
Enantioselective [2+2] Cycloadditions of Cinnamate Esters: Generalizing Lewis Acid Catalysis of Triplet Energy Transfer.J Am Chem Soc. 2019 Jun 19;141(24):9543-9547. doi: 10.1021/jacs.9b04643. Epub 2019 Jun 7. J Am Chem Soc. 2019. PMID: 31145856 Free PMC article.
-
Lewis Acid-Promoted [2 + 2] Cycloadditions of Allenes and Ketenes: Versatile Methods for Natural Product Synthesis.Acc Chem Res. 2023 Aug 15;56(16):2253-2264. doi: 10.1021/acs.accounts.3c00334. Epub 2023 Aug 4. Acc Chem Res. 2023. PMID: 37540783 Free PMC article.
-
Pathway-divergent coupling of alkynes and cyclobutenes through enantioselective cobalt catalysis.Nat Commun. 2025 Jul 1;16(1):5995. doi: 10.1038/s41467-025-61019-2. Nat Commun. 2025. PMID: 40595530 Free PMC article.
-
Tandem Isomerization/α,β-Site-Selective and Enantioselective Addition Reactions of N-(3-Butynoyl)-3,5-dimethylpyrazole Induced by Chiral π-Cu(II) Catalysts.J Am Chem Soc. 2023 Dec 13;145(49):27080-27088. doi: 10.1021/jacs.3c10820. Epub 2023 Nov 30. J Am Chem Soc. 2023. PMID: 38032102 Free PMC article.
-
Lessons in Strain and Stability: Enantioselective Synthesis of (+)-[5]-Ladderanoic Acid.Angew Chem Int Ed Engl. 2020 Jan 2;59(1):436-441. doi: 10.1002/anie.201910901. Epub 2019 Nov 18. Angew Chem Int Ed Engl. 2020. PMID: 31650679 Free PMC article.
References
-
- Diels O; Alder K Über die Ursachen der Azoesterreaktion. Justus Liebigs Ann. Chem 1926, 450, 237.
- Diels O; Alder K Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem 1928, 460, 98.
- Nicolaou KC; Snyder SA; Montagnon T; Vassilikogiannakis G The Diels–Alder Reaction in Total Synthesis. Angew. Chem., Int. Ed 2002, 41, 1668. - PubMed
-
- Woodward RB; Hoffmann R The Conservation of Orbital Symmetry. Angew. Chem., Int. Ed. Engl 1969, 8, 781.
- Fleming I Molecular Orbitals and Organic Chemical Reactions; Wiley: Chichester, 2009.
-
-
For reviews, see:
Kagan HB; Riant O Catalytic asymmetric Diels Alder reactions. Chem. Rev 1992, 92, 1007.
Corey EJ Catalytic Enantioselective Diels–Alder Reactions: Methods, Mechanistic Fundamentals, Pathways, and Applications. Angew. Chem., Int. Ed 2002, 41, 1650.
-
-
-
For recent reviews about enantioselective arylcyclobutane syntheses, see:
Brimioulle R; Lenhart D; Maturi MM; Bach T Enantioselective Catalysis of Photochemical Reactions. Angew. Chem., Int. Ed 2015, 54, 3872.
Xu Y; Conner ML; Brown MK Cyclobutane and Cyclobutene Synthesis: Catalytic Enantioselective [2+2] Cycloadditions. Angew. Chem., Int. Ed 2015, 54, 11918.
Poplata S; Tröster A; Zou Y-Q; Bach T Recent Advances in the Synthesis of Cyclobutanes by Olefin [2+2] Photocycloaddition Reactions. Chem. Rev 2016, 116, 9748.
Fructos MR; Prieto A [2+2] Cycloaddition reactions promoted by group 11 metal-based catalysts. Tetrahedron 2016, 72, 355.
Wang M; Lu P Catalytic approaches to assemble cyclobutane motifs in natural product synthesis. Org. Chem. Front 2018, 5, 254.
Brenninger C; Jolliffe JD; Bach T Chromophore Activation of α,β-Unsaturated Carbonyl Compounds and Its Application to Enantioselective Photochemical Reactions. Angew. Chem., Int. Ed 2018, 57, 14338.
-
-
-
For recent examples utilizing transition metal catalysis, see:
Kumar R; Tamai E; Ohnishi A; Nishimura A; Hoshimoto Y; Ohashi M; Ogoshi S Nickel-Catalyzed Enantioselective Synthesis of Cyclobutenes via [2+2] Cycloaddition of α,β-Unsaturated Carbonyls with 1,3-Enynes. Synthesis 2016, 48, 2789.
Kossler D; Cramer N Neutral chiral cyclopentadienyl Ru(II)Cl catalysts enable enantioselective [2+2]-cycloadditions. Chem. Sci 2017, 8, 1862.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

