Enantioselective Synthesis of a Cyclopropane Derivative of Spliceostatin A and Evaluation of Bioactivity
- PMID: 30394756
- PMCID: PMC6519444
- DOI: 10.1021/acs.orglett.8b03228
Enantioselective Synthesis of a Cyclopropane Derivative of Spliceostatin A and Evaluation of Bioactivity
Abstract
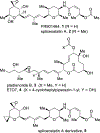
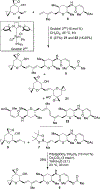
Spliceostatin A is a potent inhibitor of spliceosomes and exhibits excellent anticancer activity against multiple human cancer cell lines. We describe here the design and synthesis of a stable cyclopropane derivative of spliceostatin A. The synthesis involved a cross-metathesis or a Suzuki cross-coupling reaction as the key step. The functionalized epoxy alcohol ring was constructed from commercially available optically active tri- O-acetyl-d-glucal. The biological properties of the cyclopropyl derivative revealed that it is active in human cells and inhibits splicing in vitro comparable to spliceostatin A.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Spliceostatins and Derivatives: Chemical Syntheses and Biological Properties of Potent Splicing Inhibitors.J Nat Prod. 2021 May 28;84(5):1681-1706. doi: 10.1021/acs.jnatprod.1c00100. Epub 2021 May 11. J Nat Prod. 2021. PMID: 33974423 Free PMC article. Review.
-
Enantioselective synthesis of spliceostatin E and evaluation of biological activity.Org Lett. 2014 Dec 5;16(23):6200-3. doi: 10.1021/ol503127r. Epub 2014 Nov 25. Org Lett. 2014. PMID: 25423085 Free PMC article.
-
Enantioselective total syntheses of FR901464 and spliceostatin A and evaluation of splicing activity of key derivatives.J Org Chem. 2014 Jun 20;79(12):5697-709. doi: 10.1021/jo500800k. Epub 2014 May 30. J Org Chem. 2014. PMID: 24873648 Free PMC article.
-
Enantioselective syntheses of FR901464 and spliceostatin A: potent inhibitors of spliceosome.Org Lett. 2013 Oct 4;15(19):5088-91. doi: 10.1021/ol4024634. Epub 2013 Sep 19. Org Lett. 2013. PMID: 24050251 Free PMC article.
-
Cephalostatin analogues--synthesis and biological activity.Fortschr Chem Org Naturst. 2004;87:1-80. doi: 10.1007/978-3-7091-0581-8_1. Fortschr Chem Org Naturst. 2004. PMID: 15079895 Review.
Cited by
-
Synthesis, antifungal activity and 3D-QSAR study of novel acyl thiourea compounds containing gem-dimethylcyclopropane ring.Mol Divers. 2022 Feb;26(1):125-136. doi: 10.1007/s11030-020-10163-6. Epub 2021 Apr 29. Mol Divers. 2022. PMID: 33914211
-
Structure-Activity Relationship Study of Splicing Modulators on Hsh155/SF3B1 through Chemical Synthesis and Yeast Genetics.ACS Med Chem Lett. 2024 Nov 25;15(12):2225-2230. doi: 10.1021/acsmedchemlett.4c00510. eCollection 2024 Dec 12. ACS Med Chem Lett. 2024. PMID: 39691513 Free PMC article.
-
Spliceostatins and Derivatives: Chemical Syntheses and Biological Properties of Potent Splicing Inhibitors.J Nat Prod. 2021 May 28;84(5):1681-1706. doi: 10.1021/acs.jnatprod.1c00100. Epub 2021 May 11. J Nat Prod. 2021. PMID: 33974423 Free PMC article. Review.
-
Total Synthesis of Meayamycin and O-Acyl Analogues.Org Lett. 2020 Nov 6;22(21):8714-8719. doi: 10.1021/acs.orglett.0c03308. Epub 2020 Oct 19. Org Lett. 2020. PMID: 33074680 Free PMC article.
-
Synthesis and Evaluation of Biological Activities of Bis(spiropyrazolone)cyclopropanes: A Potential Application against Leishmaniasis.Molecules. 2021 Aug 17;26(16):4960. doi: 10.3390/molecules26164960. Molecules. 2021. PMID: 34443548 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

