Protein kinase C binding protein 1 inhibits hypoxia-inducible factor-1 in the heart
- PMID: 30395227
- PMCID: PMC6587917
- DOI: 10.1093/cvr/cvy278
Protein kinase C binding protein 1 inhibits hypoxia-inducible factor-1 in the heart
Abstract
Aims: Hypoxia-inducible factor-1 alpha (HIF-1α) is a key transcription factor responsible for the induction of genes that facilitate adaptation to hypoxia. To study HIF-1 signalling in the heart, we developed a mouse model in which an oxygen-stable form of HIF-1α can be inducibly expressed in cardiac myocytes, under the regulation of tetracycline.
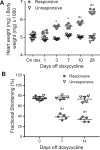
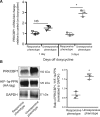
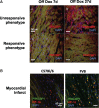
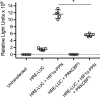
Methods and results: Remarkably, expression of the transgene in mice generated two distinct phenotypes. One was the expected expression of HIF-regulated transcripts and associated changes in cardiac angiogenesis and contractility. The other was an unresponsive phenotype with much less expression of typical HIF-response genes and substantial expression of a zinc-finger protein, Protein Kinase C Binding Protein 1 (PRKCBP1). We have demonstrated that this second phenotype is due to an insertion of a fragment of DNA upstream of the PRKCBP1 gene that contains two additional canonical HIF binding sites and leads to substantial HIF binding, assessed by chromatin immunoprecipitation, and transcriptional activation. This insertion is found only in the FVB strain of mice that contributed the αMHC-tet binding protein transgene to these biallelic mice. In HEK293 cells transfected with oxygen-stable HIF-1α and PRKCBP1, we demonstrated inhibition of HIF-1 activity by a luciferase reporter assay. Using mouse primary cells and cell lines, we show that transfection with oxygen-stable HIF-1α and PRKCBP1 reduced expression of direct HIF-1 gene targets and that knockdown of PRKCBP1 removes that negative inhibition. Consistent with previous reports suggesting that PRKCBP1 modulates the chromatin landscape, we found that HL-1 cells transfected with oxygen-stable HIF-1α and PRKCBP1 have reduced global 5-methyl cytosine compared to HIF-1 alone.
Conclusion: We show genetic, transcriptional, biochemical, and physiological evidence that PRKCBP1 inhibits HIF activity. Identification of a new oxygen-dependent and previously unsuspected regulator of HIF may provide a target for new therapeutic approaches to ischaemic heart disease.
Keywords: Genetic variance; HIF inhibition; RACK7; Zmynd8; transcriptional repressor.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2018. For permissions, please email: journals.permissions@oup.com.
Figures


Comment in
-
The many facets of transcriptional regulation by hypoxia-inducible factor-1.Cardiovasc Res. 2019 Jul 1;115(8):1257-1259. doi: 10.1093/cvr/cvz079. Cardiovasc Res. 2019. PMID: 30895323 No abstract available.
References
-
- Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 1998;8:588–594. - PubMed
-
- Shohet RV, Garcia JA.. Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia. J Mol Med 2007;85:1309–1315. - PubMed
-
- Masson N, Ratcliffe PJ.. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J Cell Sci 2003;116:3041–3049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

