FAM92A Underlies Nonsyndromic Postaxial Polydactyly in Humans and an Abnormal Limb and Digit Skeletal Phenotype in Mice
- PMID: 30395363
- PMCID: PMC6489482
- DOI: 10.1002/jbmr.3594
FAM92A Underlies Nonsyndromic Postaxial Polydactyly in Humans and an Abnormal Limb and Digit Skeletal Phenotype in Mice
Abstract
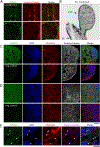
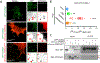
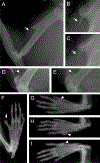
Polydactyly is a common congenital anomaly of the hand and foot. Postaxial polydactyly (PAP) is characterized by one or more posterior or postaxial digits. In a Pakistani family with autosomal recessive nonsyndromic postaxial polydactyly type A (PAPA), we performed genomewide genotyping, linkage analysis, and exome and Sanger sequencing. Exome sequencing revealed a homozygous nonsense variant (c.478C>T, p.[Arg160*]) in the FAM92A gene within the mapped region on 8q21.13-q24.12 that segregated with the PAPA phenotype. We found that FAM92A is expressed in the developing mouse limb and E11.5 limb bud including the progress zone and the apical ectodermal ridge, where it strongly localizes at the cilia level, suggesting an important role in limb patterning. The identified variant leads to a loss of the FAM92A/Chibby1 complex that is crucial for ciliogenesis and impairs the recruitment and the colocalization of FAM92A with Chibby1 at the base of the cilia. In addition, we show that Fam92a-/- homozygous mice also exhibit an abnormal digit morphology, including metatarsal osteomas and polysyndactyly, in addition to distinct abnormalities on the deltoid tuberosity of their humeri. In conclusion, we present a new nonsyndromic PAPA ciliopathy due to a loss-of-function variant in FAM92A. © 2018 American Society for Bone and Mineral Research.
Keywords: CHIBBY1; CILIOPATHY; FAM92A; PAPA; POSTAXIAL POLYDACTYLY.
© 2018 American Society for Bone and Mineral Research.
Conflict of interest statement
Disclosures
All authors state that they have no conflicts of interest.
Figures



References
-
- Castilla EE, Lugarinho R, da Graça Dutra M, Salgado LJ. Associated anomalies in individuals with polydactyly. Am J Med Genet. 1998;80(5):459–65. - PubMed
-
- Galjaard R-JH, Smits APT, Tuerlings JHAM, et al. A new locus for postaxial polydactyly type A/Bon chromosome 7q21-q34. Eur J Hum Genet. 2003;11(5):409–15. - PubMed
-
- Zhao H, Tian Y, Breedveld G, et al. Postaxial polydactyly type A/B (PAP-A/B) is linked to chromosome 19p13.1–13.2 in a Chinese kindred. Eur J Hum Genet. 2002;10(3):162–6. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

