Deregulation of the imprinted DLK1-DIO3 locus ncRNAs is associated with replicative senescence of human adipose-derived stem cells
- PMID: 30395586
- PMCID: PMC6218046
- DOI: 10.1371/journal.pone.0206534
Deregulation of the imprinted DLK1-DIO3 locus ncRNAs is associated with replicative senescence of human adipose-derived stem cells
Abstract
Background: Human adult adipose-derived stem cells (hADSCs) have become the most promising cell source for regenerative medicine. However the prolonged ex vivo expansion periods required to obtain the necessary therapeutic dose promotes progressive senescence, with the concomitant reduction of their therapeutic potential.
Aim and scope: A better understanding of the determinants of hADSC senescence is needed to improve biosafety while preserving therapeutic efficiency. Here, we investigated the association between deregulation of the imprinted DLK1-DIO3 region and replicative senescence in hADSC cultures.
Methods: We compared hADSC cultures at short (PS) and prolonged (PL) passages, both in standard and low [O2] (21 and 3%, respectively), in relation to replicative senescence. hADSCs were evaluated for expression alterations in the DLK1-DIO3 region on chromosome 14q32, and particularly in its main miRNA cluster.
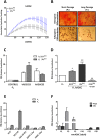
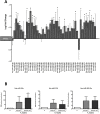
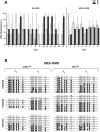
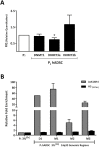
Results: Comparison of hADSCs cultured at PL or PS surprisingly showed a quite significant fraction (69%) of upregulated miRNAs in PL cultures mapping to the imprinted 14q32 locus, the largest miRNA cluster described in the genome. In agreement, expression of the lncRNA MEG3 (Maternally Expressed 3; Meg3/Gtl2), cultured at 21 and 3% [O2], was also significantly higher in PL than in PS passages. During hADSC replicative senescence the AcK16H4 activating mark was found to be significantly associated with the deregulation of the entire DLK1-DIO3 locus, with a secondary regulatory role for the methylation of DMR regions.
Conclusion: A direct relationship between DLK1-DIO3 deregulation and replicative senescence of hADSCs is reported, involving upregulation of a very significant fraction of its largest miRNA cluster (14q32.31), paralleled by the progressive overexpression of the lncRNA MEG3, which plays a central role in the regulation of Dlk1/Dio3 activation status in mice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Loss of non-coding RNA expression from the DLK1-DIO3 imprinted locus correlates with reduced neural differentiation potential in human embryonic stem cell lines.Stem Cell Res Ther. 2015 Jan 5;6(1):1. doi: 10.1186/scrt535. Stem Cell Res Ther. 2015. PMID: 25559585 Free PMC article.
-
Dlk1-Dio3 locus-derived lncRNAs perpetuate postmitotic motor neuron cell fate and subtype identity.Elife. 2018 Oct 12;7:e38080. doi: 10.7554/eLife.38080. Elife. 2018. PMID: 30311912 Free PMC article.
-
Male-specific coordinated changes in expression of miRNA genes, but not other genes within the DLK1-DIO3 locus in multiple sclerosis.Gene. 2022 Aug 20;836:146676. doi: 10.1016/j.gene.2022.146676. Epub 2022 Jun 14. Gene. 2022. PMID: 35714798
-
DLK1-DIO3 imprinted locus deregulation in development, respiratory disease, and cancer.Expert Rev Respir Med. 2017 Sep;11(9):749-761. doi: 10.1080/17476348.2017.1355241. Epub 2017 Jul 20. Expert Rev Respir Med. 2017. PMID: 28715922 Review.
-
The microRNAs within the DLK1-DIO3 genomic region: involvement in disease pathogenesis.Cell Mol Life Sci. 2013 Mar;70(5):795-814. doi: 10.1007/s00018-012-1080-8. Epub 2012 Jul 24. Cell Mol Life Sci. 2013. PMID: 22825660 Free PMC article. Review.
Cited by
-
Aging and Metabolic Reprogramming of Adipose-Derived Stem Cells Affect Molecular Mechanisms Related to Cardiovascular Diseases.Cells. 2023 Dec 7;12(24):2785. doi: 10.3390/cells12242785. Cells. 2023. PMID: 38132104 Free PMC article. Review.
-
Maternally expressed gene 3 in metabolic programming.Biochim Biophys Acta Gene Regul Mech. 2020 Apr;1863(4):194396. doi: 10.1016/j.bbagrm.2019.06.007. Epub 2019 Jul 1. Biochim Biophys Acta Gene Regul Mech. 2020. PMID: 31271897 Free PMC article. Review.
-
The hippo pathway: a molecular bridge between environmental cues and pace of life.BMC Ecol Evol. 2025 Apr 24;25(1):35. doi: 10.1186/s12862-025-02378-8. BMC Ecol Evol. 2025. PMID: 40275190 Free PMC article. Review.
-
Comprehensive Assessment of Copy Number Alterations Uncovers Recurrent AIFM3 and DLK1 Copy Gain in Medullary Thyroid Carcinoma.Cancers (Basel). 2021 Jan 9;13(2):218. doi: 10.3390/cancers13020218. Cancers (Basel). 2021. PMID: 33435319 Free PMC article.
-
Long Non-Coding RNA MEG3 in Cellular Stemness.Int J Mol Sci. 2021 May 19;22(10):5348. doi: 10.3390/ijms22105348. Int J Mol Sci. 2021. PMID: 34069546 Free PMC article. Review.
References
-
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003; 116:1827–35. - PubMed

