Economic and epidemiologic impact of guidelines for early ART initiation irrespective of CD4 count in Spain
- PMID: 30395635
- PMCID: PMC6218062
- DOI: 10.1371/journal.pone.0206755
Economic and epidemiologic impact of guidelines for early ART initiation irrespective of CD4 count in Spain
Abstract
Introduction: Emerging data suggest that early antiretroviral therapy (ART) could reduce serious AIDS and non-AIDS events and deaths but could also increase costs. In January 2016, the Spanish guidelines were updated to recommend ART at any CD4 count. However, the epidemiologic and economic impacts of early ART initiation in Spain remain unclear.
Methods: The Johns Hopkins HIV Economic-Epidemiologic Mathematical Model (JHEEM) was utilized to estimate costs, transmissions, and outcomes in Spain over 20 years. We compared implementation of guidelines for early ART initiation to a counterfactual scenario deferring ART until CD4-counts fall below 350 cells/mm3. We additionally studied the impact of early ART initiation in combination with improvements to HIV screening, care linkage and engagement.
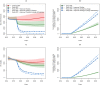
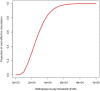
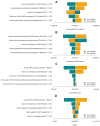
Results: Early ART initiation (irrespective of CD4-count) is expected to avert 20,100 [95% Uncertainty Range (UR) 11,100-83,000] new HIV cases over the next two decades compared to delayed ART (28% reduction), at an incremental health system cost of €1.05 billion [€0.66 - €1.63] billion, and an incremental cost-effectiveness ratio (ICER) of €29,700 [€13,700 - €41,200] per QALY gained. Projected ICERs declined further over longer time horizon; e.g., an ICER of €12,691 over 30 years. Furthermore, the impact of early ART initiation was potentiated by improved HIV screening among high-risk individuals, averting an estimated 41,600 [23,200-172,200] HIV infections (a 58% decline) compared to delayed ART.
Conclusions: Recommendations for ART initiation irrespective of CD4-counts are cost-effective and could avert > 30% of new cases in Spain. Improving HIV diagnosis can amplify this impact.
Conflict of interest statement
MR and BH are employees of ViiV Healthcare Inc; MS and PK were supported through a research grant from ViiV Healthcare Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials. All other authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures
Similar articles
-
Cost-effectiveness and budget impact of immediate antiretroviral therapy initiation for treatment of HIV infection in Côte d'Ivoire: A model-based analysis.PLoS One. 2019 Jun 27;14(6):e0219068. doi: 10.1371/journal.pone.0219068. eCollection 2019. PLoS One. 2019. PMID: 31247009 Free PMC article.
-
When to start antiretroviral therapy in resource-limited settings.Ann Intern Med. 2009 Aug 4;151(3):157-66. doi: 10.7326/0003-4819-151-3-200908040-00138. Epub 2009 Jul 20. Ann Intern Med. 2009. PMID: 19620143 Free PMC article.
-
Optimal frequency of CD4 cell count and HIV RNA monitoring prior to initiation of antiretroviral therapy in HIV-infected patients.Antivir Ther. 2005;10(1):41-52. Antivir Ther. 2005. PMID: 15751762
-
[Consensus document of Gesida and Spanish Secretariat for the National Plan on AIDS (SPNS) regarding combined antiretroviral treatment in adults infected by the human immunodeficiency virus (January 2012)].Enferm Infecc Microbiol Clin. 2012 Jun;30(6):e1-89. doi: 10.1016/j.eimc.2012.03.006. Epub 2012 May 23. Enferm Infecc Microbiol Clin. 2012. PMID: 22633764 Spanish.
-
[Recommendations from the GESIDA/Spanish AIDS Plan regarding antiretroviral treatment in adults with human immunodeficiency virus infection (update February 2009)].Enferm Infecc Microbiol Clin. 2009 Apr;27(4):222-35. doi: 10.1016/j.eimc.2008.11.002. Epub 2009 Feb 26. Enferm Infecc Microbiol Clin. 2009. PMID: 19246124 Spanish.
Cited by
-
Cost-Effectiveness of HIV Retention and Re-engagement Interventions in High-Income Countries: A Systematic Literature Review.AIDS Behav. 2022 Jul;26(7):2159-2168. doi: 10.1007/s10461-021-03561-w. Epub 2022 Jan 25. AIDS Behav. 2022. PMID: 35076798 Free PMC article.
-
Epidemiologic and Economic Analysis of Rapid Antiretroviral Therapy Initiation with Bictegravir/Emtricitabine/Tenofovir Alafenamide in Spain.Pharmacoecon Open. 2022 May;6(3):415-424. doi: 10.1007/s41669-022-00322-w. Epub 2022 Feb 5. Pharmacoecon Open. 2022. PMID: 35124787 Free PMC article.
-
An Online HIV Self-Sampling Strategy for Gay, Bisexual and Other Men Who Have Sex with Men and Trans Women in Spain.J Community Health. 2024 Jun;49(3):535-548. doi: 10.1007/s10900-023-01311-8. Epub 2023 Dec 23. J Community Health. 2024. PMID: 38141149 Free PMC article.
-
Real-world clinical and economic outcomes from rapid start antiretroviral therapy in HIV: systematic review and meta-analysis.AIDS. 2025 Mar 1;39(3):241-252. doi: 10.1097/QAD.0000000000004046. Epub 2024 Oct 24. AIDS. 2025. PMID: 39453866 Free PMC article.
-
Implicit bias in HIV testing based on indicator conditions in primary care: a population-based study in Catalonia, Spain, 2017 to 2021.Euro Surveill. 2025 Jun;30(24):2400585. doi: 10.2807/1560-7917.ES.2025.30.24.2400585. Euro Surveill. 2025. PMID: 40539311 Free PMC article.
References
-
- HIV/AIDS JUNPo. Global AIDS update 2016 2016 [03/16/2018]. Available from: http://www.unaids.org/sites/default/Files/media_asset/global-AIDS-update....
-
- Roser M. HIV / AIDS 2017 [updated 02/08/201803/16/2018]. Available from: https://ourworldindata.org/hiv-aids.
-
- WHO. Scaling up antiretroviral therapy in resource-limited settings. 2002 2002 [03/16/2018]. Available from: http://www.who.int/hiv/pub/prev_care/en/ScalingUp_E.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

