Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension
- PMID: 30395897
- PMCID: PMC6290172
- DOI: 10.1016/j.jconrel.2018.11.002
Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension
Abstract
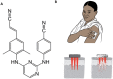
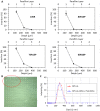
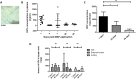
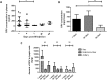
One means of combating the spread of human immunodeficiency virus (HIV) is through the delivery of long-acting, antiretroviral (ARV) drugs for prevention and treatment. The development of a discreet, self-administered and self-disabling delivery vehicle to deliver such ARV drugs could obviate compliance issues with daily oral regimens. Alternatives in development, such as long-acting intramuscular (IM) injections, require regular access to health care facilities and disposal facilities for sharps. Consequently, this proof of concept study was developed to evaluate the use of dissolving microarray patches (MAPs) containing a long-acting (LA) nanosuspension of the candidate ARV drug, rilpivirine (RPV). MAPs were mechanically strong and penetrated skin in vitro, delivering RPV intradermally. In in vivo studies, the mean plasma concentration of RPV in rats (431 ng/ml at the Day 7 time point) was approximately ten-fold greater than the trough concentration observed after a single-dose in previous clinical studies. These results are the first to indicate, by the determination of relative exposures between IM and MAP administration, that larger multi-array dissolving MAPs could potentially be used to effectively deliver human doses of RPV LA. Importantly, RPV was also detected in the lymph nodes, indicating the potential to deliver this ARV agent into one of the primary sites of HIV replication over extended durations. These MAPs could potentially improve patient acceptability and adherence to HIV prevention and treatment regimens and combat instances of needle-stick injury and the transmission of blood-borne diseases, which would have far-reaching benefits, particularly to those in the developing world.
Keywords: Antiretroviral; HIV; Microarray patch; Rilpivirine.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Public Health England HIV diagnoses, late diagnoses and numbers accessing treatment and care. Version. 2016;1(0):4–8.
-
- Mosam A., Dlova N.C. HIV/AIDS in sub-Saharan Africa. Dermatol. Clin. 2006;24(4):421–429. - PubMed
-
- Sawers L., Isaac A. Partnership duration, concurrency, and HIV in sub-Saharan Africa. Afr. J. AIDS Res. 2017;17:1–10. - PubMed
-
- Nyaku A.N., Kelly S.G., Taiwo B.O. Long-acting antiretrovirals: where are we now? Curr. HIV/AIDS Rep. 2017;14(2):63–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

