Formulation of aripiprazole-loaded pH-modulated solid dispersions via hot-melt extrusion technology: In vitro and in vivo studies
- PMID: 30395959
- PMCID: PMC6312482
- DOI: 10.1016/j.ijpharm.2018.11.005
Formulation of aripiprazole-loaded pH-modulated solid dispersions via hot-melt extrusion technology: In vitro and in vivo studies
Abstract
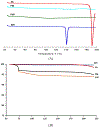
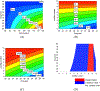
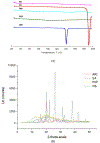
The objective of this study was to formulate aripiprazole (ARI)-loaded pH-modulated solid dispersions (SD) to enhance solubility, dissolution, and bioavailability via hot-melt extrusion (HME) technology. Kollidon® 12 PF (PVP) and succinic acid (SA) were selected after solubility screenings of various polymers and acidifiers. Several formulations, varying in screw speed and drug/polymer/acidifier ratios, were extruded using an 11 mm twin-screw extruder and were investigated for the effect of these variables. Scanning electron microscopy (SEM), differential scanning calorimetry (DSC), and X-ray diffraction (XRD) were used to perform solid-state characterizations of the pure drug and extrudates. The aqueous solubility and dissolution were evaluated for the pure drug and milled extrudates. Among the prepared formulations, N6 was chosen for in vivo absorption studies. Solid-state characterization demonstrated the transformation of the crystalline ARI to an amorphous state in the formulations. Each formulation showed increased solubility and dissolution compared to the drug powder. The oral bioavailability (Cmax and AUC0-12) of N6 was significantly improved when compared to the pure ARI. This novel study not only discusses the incorporation of acidifiers in SDs but also the preparation of SDs using HME technology as effective techniques to improve drug release and bioavailability.
Keywords: Acidifier; Aripiprazole; Hot-melt extrusion; Oral bioavailability; Pharmacokinetics; Solid dispersion.
Published by Elsevier B.V.
Conflict of interest statement
Disclosure
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Preparation, characterization and in vitro/vivo evaluation of tectorigenin solid dispersion with improved dissolution and bioavailability.Eur J Drug Metab Pharmacokinet. 2016 Aug;41(4):413-22. doi: 10.1007/s13318-015-0265-6. Epub 2015 Feb 11. Eur J Drug Metab Pharmacokinet. 2016. PMID: 25669445
-
Soluplus®/TPGS-based solid dispersions prepared by hot-melt extrusion equipped with twin-screw systems for enhancing oral bioavailability of valsartan.Drug Des Devel Ther. 2015 May 22;9:2745-56. doi: 10.2147/DDDT.S84070. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26045660 Free PMC article.
-
A New Extrudable Form of Hypromellose: AFFINISOL™ HPMC HME.AAPS PharmSciTech. 2016 Feb;17(1):106-19. doi: 10.1208/s12249-015-0395-9. Epub 2015 Sep 4. AAPS PharmSciTech. 2016. PMID: 26335416 Free PMC article.
-
Navigating the Solution to Drug Formulation Problems at Research and Development Stages by Amorphous Solid Dispersion Technology.Recent Adv Drug Deliv Formul. 2024;18(2):79-99. doi: 10.2174/0126673878271641231201065151. Recent Adv Drug Deliv Formul. 2024. PMID: 38062659 Review.
-
Hot-Melt Extrusion: a Roadmap for Product Development.AAPS PharmSciTech. 2021 Jun 17;22(5):184. doi: 10.1208/s12249-021-02017-7. AAPS PharmSciTech. 2021. PMID: 34142250 Review.
Cited by
-
Developing HME-Based Drug Products Using Emerging Science: a Fast-Track Roadmap from Concept to Clinical Batch.AAPS PharmSciTech. 2020 Jun 22;21(5):176. doi: 10.1208/s12249-020-01713-0. AAPS PharmSciTech. 2020. PMID: 32572701 Free PMC article. Review.
-
Curcumin Plant for Colorectal Cancer Prediction and Prevention Using In Silico Molecular Analysis; HOT-MELT Extrusion.Evid Based Complement Alternat Med. 2022 Jun 23;2022:4376960. doi: 10.1155/2022/4376960. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Aug 9;2023:9836859. doi: 10.1155/2023/9836859. PMID: 35783520 Free PMC article. Retracted.
-
Towards predicting the product quality in hot-melt extrusion: Small scale extrusion.Int J Pharm X. 2020 Nov 19;2:100062. doi: 10.1016/j.ijpx.2020.100062. eCollection 2020 Dec. Int J Pharm X. 2020. PMID: 33299982 Free PMC article.
-
Coupling hot melt extrusion and fused deposition modeling: Critical properties for successful performance.Adv Drug Deliv Rev. 2021 May;172:52-63. doi: 10.1016/j.addr.2021.02.006. Epub 2021 Feb 9. Adv Drug Deliv Rev. 2021. PMID: 33571550 Free PMC article. Review.
-
Experimental solubility of aripiprazole in supercritical carbon dioxide and modeling.Sci Rep. 2023 Aug 17;13(1):13402. doi: 10.1038/s41598-023-40537-3. Sci Rep. 2023. PMID: 37591914 Free PMC article.
References
-
- Borbás E, Balogh A, Bocz K, Müller J, Kiserdei É, Vigh T, Sinkó B, Marosi A, Halász A, Dohányos Z, Szente L, Balogh GT, Nagy ZK, 2015. In vitro dissolution–permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using μ Flux™. International Journal of Pharmaceutics. 491, 180–189. 10.1016/j.ijpharm.2015.06.019 - DOI - PubMed
-
- Chi L, Liu R, Guo T, Wang M, Liao Z, Wu L, Li H, Wu D, Zhang J, 2015. Dramatic improvement of the solubility of pseudolaric acid B by cyclodextrin complexation: preparation, characterization and validation. International Journal of Pharmaceutics. 479, 349–356. 10.1016/j.ijpharm.2015.01.005 - DOI - PubMed
-
- Daravath B, Tadikonda RR, Vemula SK, 2015. Formulation and pharmacokinetics of gelucire solid dispersions of flurbiprofen. Drug Development and Industrial Pharmacy. 41, 1254–1262. https://doi.org10.3109/03639045.2014.940963 - PubMed
-
- Fousteris E, Tarantili PA, Karavas E, Bikiaris D, 2013. Poly(vinyl pyrrolidone)-poloxamer-188 solid dispersions prepared by hot melt extrusion. Journal of Thermal Analysis & Calorimetry. 113, 1037–1047. 10.1007/s10973-012-2885-2 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

