Simultaneous LC-MS/MS analysis of simvastatin, atorvastatin, rosuvastatin and their active metabolites for plasma samples of obese patients underwent gastric bypass surgery
- PMID: 30396053
- PMCID: PMC6298799
- DOI: 10.1016/j.jpba.2018.10.045
Simultaneous LC-MS/MS analysis of simvastatin, atorvastatin, rosuvastatin and their active metabolites for plasma samples of obese patients underwent gastric bypass surgery
Abstract
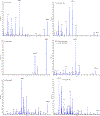
Statins, HMG-CoA reductase inhibitors, are considered the first line treatment of hyperlipidemia to reduce the risk of atherosclerotic cardiovascular diseases. The prevalence of hyperlipidemia and the risk of atherosclerotic cardiovascular diseases are higher in obese patients. Published methods for the quantification of statins and their active metabolites did not test for matrix effect of or validate the method in hyperlipidemic plasma. A sensitive, specific, accurate, and reliable LC-MS/MS method for the simultaneous quantification of simvastatin (SMV), active metabolite of simvastatin acid (SMV-A), atorvastatin (ATV), active metabolites of 2-hydroxy atorvastatin (2-OH-ATV), 4-hydroxy atorvastatin (4-OH-ATV), and rosuvastatin (RSV) was developed and validated in plasma with low (52-103 mg/dl, <300 mg/dl) and high (352-403 mg/dl, >300 mg/dl) levels of triglyceride. The column used in this method was ACQUITY UPLC BEH C18 column (2.1 × 100 mm I.D., 1.7 μm). A gradient elution of mobile phase A (10 mM ammonium formate and 0.04% formic acid in water) and mobile phase B (acetonitrile) was used with a flow rate of 0.4 ml/min and run time of 5 min. The transitions of m/z 436.3 → 285.2 for SMV, m/z 437.2 → 303.2 for SMV-A, m/z 559.2 → 440.3 for ATV, m/z 575.4 → 440.3 for 2-OH-ATV and 4-OH-ATV, m/z 482.3 → 258.1 for RSV, and m/z 412.3 → 224.2 for fluvastatin (internal standard, IS) were determined by Selected Reaction Monitoring (SRM) method to detect transitions ions in the positive ion mode. The assay has a linear range of 0.25 (LLOQ) -100 ng/ml for all six analytes. Accuracy (87-114%), precision (3-13%), matrix effect (92-110%), and extraction recovery (88-100%) of the assay were within the 15% acceptable limit of FDA Guidelines in variations for plasma with both low and high triglyceride levels. The method was used successfully for the quantification of SMV, ATV, RSV, and their active metabolites in human plasma samples collected for an ongoing clinical pharmacokinetic and pharmacodynamic study on patients prior to and post gastric bypass surgery (GBS).
Keywords: Atorvastatin and metabolites; Hyperlipidemic plasma; LC–MS/MS; Obese subjects from GBS; Rosuvastatin; Simvastatin and metabolite.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Liquid chromatography-tandem mass spectrometry assay for the simultaneous quantification of simvastatin, lovastatin, atorvastatin, and their major metabolites in human plasma.J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Mar 1;983-984:18-25. doi: 10.1016/j.jchromb.2014.12.029. Epub 2015 Jan 13. J Chromatogr B Analyt Technol Biomed Life Sci. 2015. PMID: 25612772 Free PMC article.
-
Simultaneous analysis of the total plasma concentration of atorvastatin and its five metabolites and the unbound plasma concentration of atorvastatin: Application in a clinical pharmacokinetic study of single oral dose.J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Sep 15;1126-1127:121766. doi: 10.1016/j.jchromb.2019.121766. Epub 2019 Aug 17. J Chromatogr B Analyt Technol Biomed Life Sci. 2019. PMID: 31450089
-
Method development for quantitative determination of seven statins including four active metabolites by means of high-resolution tandem mass spectrometry applicable for adherence testing and therapeutic drug monitoring.Clin Chem Lab Med. 2020 Apr 28;58(5):664-672. doi: 10.1515/cclm-2019-0763. Clin Chem Lab Med. 2020. PMID: 31665111
-
Analysis of five HMG-CoA reductase inhibitors-- atorvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin: pharmacological, pharmacokinetic and analytical overview and development of a new method for use in pharmaceutical formulations analysis and in vitro metabolism studies.Biomed Chromatogr. 2006 Mar;20(3):282-93. doi: 10.1002/bmc.561. Biomed Chromatogr. 2006. PMID: 16143964 Review.
-
The effect of local and systemic statin use as an adjunct to non-surgical and surgical periodontal therapy-A systematic review and meta-analysis.J Dent. 2017 Dec;67:18-28. doi: 10.1016/j.jdent.2017.08.011. J Dent. 2017. PMID: 28855141
Cited by
-
Identification and Functional Characterization of Metabolites for Bone Mass in Peri- and Postmenopausal Chinese Women.J Clin Endocrinol Metab. 2021 Jul 13;106(8):e3159-e3177. doi: 10.1210/clinem/dgab146. J Clin Endocrinol Metab. 2021. PMID: 33693744 Free PMC article.
-
A rapid method for determination of rosuvastatin in blood plasma with supported liquid extraction.J Mass Spectrom Adv Clin Lab. 2025 Apr 10;36:29-36. doi: 10.1016/j.jmsacl.2025.04.003. eCollection 2025 Apr. J Mass Spectrom Adv Clin Lab. 2025. PMID: 40264814 Free PMC article.
-
Development of an LC-MS method for the determination of simvastatin and its hydroxy acid form in muscle tissue and method application.PLoS One. 2025 May 5;20(5):e0322808. doi: 10.1371/journal.pone.0322808. eCollection 2025. PLoS One. 2025. PMID: 40323990 Free PMC article.
-
Longitudinal Impacts of Gastric Bypass Surgery on Pharmacodynamics and Pharmacokinetics of Statins.Obes Surg. 2019 Aug;29(8):2571-2583. doi: 10.1007/s11695-019-03885-6. Obes Surg. 2019. PMID: 31004269 Free PMC article. Clinical Trial.
-
A pharmacokinetics-based approach to the monitoring of patient adherence to atorvastatin therapy.Pharmacol Res Perspect. 2021 Oct;9(5):e00856. doi: 10.1002/prp2.856. Pharmacol Res Perspect. 2021. PMID: 34478238 Free PMC article.
References
-
- Shitara Y and Sugiyama Y, Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther, 2006. 112(1): p. 71–105. - PubMed
-
- Stone NJ, et al., 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014. 129(25 Suppl 2): p. S1–45. - PubMed
-
- Feingold KR and Grunfeld C, Obesity and Dyslipidemia, in Endotext LJ De Groot, et al., Editors. 2000: South Dartmouth (MA).
-
- Rifai N, Merrill JR, and Holly RG, Postprandial effect of a high fat meal on plasma lipid, lipoprotein cholesterol and apolipoprotein measurements. Ann Clin Biochem, 1990. 27 (Pt 5): p. 489–93. - PubMed
-
- Ismaiel OA, et al., Investigation of endogenous blood plasma phospholipids, cholesterol and glycerides that contribute to matrix effects in bioanalysis by liquid chromatography/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 2010. 878(31): p. 3303–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

