Shorter Phosphorodiamidate Morpholino Splice-Switching Oligonucleotides May Increase Exon-Skipping Efficacy in DMD
- PMID: 30396145
- PMCID: PMC6222172
- DOI: 10.1016/j.omtn.2018.10.002
Shorter Phosphorodiamidate Morpholino Splice-Switching Oligonucleotides May Increase Exon-Skipping Efficacy in DMD
Abstract
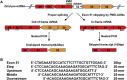
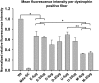
Duchenne muscular dystrophy is a fatal muscle disease, caused by mutations in DMD, leading to loss of dystrophin expression. Phosphorodiamidate morpholino splice-switching oligonucleotides (PMO-SSOs) have been used to elicit the restoration of a partially functional truncated dystrophin by excluding disruptive exons from the DMD messenger. The 30-mer PMO eteplirsen (EXONDYS51) developed for exon 51 skipping is the first dystrophin-restoring, conditionally FDA-approved drug in history. Clinical trials had shown a dose-dependent variable and patchy dystrophin restoration. The main obstacle for efficient dystrophin restoration is the inadequate uptake of PMOs into skeletal muscle fibers at low doses. The excessive cost of longer PMOs has limited the utilization of higher dosing. We designed shorter 25-mer PMOs directed to the same eteplirsen-targeted region of exon 51 and compared their efficacies in vitro and in vivo in the mdx52 murine model. Our results showed that skipped-dystrophin induction was comparable between the 30-mer PMO sequence of eteplirsen and one of the shorter PMOs, while the other 25-mer PMOs showed lower exon-skipping efficacies. Shorter PMOs would make higher doses economically feasible, and high dosing would result in better drug uptake into muscle, induce higher levels of dystrophin restoration in DMD muscle, and, ultimately, increase the clinical efficacy.
Keywords: DMD; PMO; dystrophin; eteplirsen; exon skipping; exondys51; mdx; myopathy; phosphorodiamidate morpholino; phosphorothiorate; shorter PMO-SSOs.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
In Vivo Evaluation of Multiple Exon Skipping with Peptide-PMOs in Cardiac and Skeletal Muscles in Dystrophic Dogs.Methods Mol Biol. 2018;1828:365-379. doi: 10.1007/978-1-4939-8651-4_23. Methods Mol Biol. 2018. PMID: 30171554
-
In Vivo Evaluation of Multiple Exon Skipping with Peptide-PMOs in Cardiac and Skeletal Muscles in Dystrophic Dogs.Methods Mol Biol. 2025;2964:329-343. doi: 10.1007/978-1-0716-4730-1_22. Methods Mol Biol. 2025. PMID: 40720029
-
In Vitro Multiexon Skipping by Antisense PMOs in Dystrophic Dog and Exon 7-Deleted DMD Patient.Methods Mol Biol. 2018;1828:151-163. doi: 10.1007/978-1-4939-8651-4_9. Methods Mol Biol. 2018. PMID: 30171540 Free PMC article.
-
Pharmacology and toxicology of eteplirsen and SRP-5051 for DMD exon 51 skipping: an update.Arch Toxicol. 2022 Jan;96(1):1-9. doi: 10.1007/s00204-021-03184-z. Epub 2021 Nov 19. Arch Toxicol. 2022. PMID: 34797383 Review.
-
Immortalized Muscle Cell Model to Test the Exon Skipping Efficacy for Duchenne Muscular Dystrophy.J Pers Med. 2017 Oct 16;7(4):13. doi: 10.3390/jpm7040013. J Pers Med. 2017. PMID: 29035327 Free PMC article. Review.
Cited by
-
A Decade of Progress in Gene Targeted Therapeutic Strategies in Duchenne Muscular Dystrophy: A Systematic Review.Front Bioeng Biotechnol. 2022 Mar 23;10:833833. doi: 10.3389/fbioe.2022.833833. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35402409 Free PMC article.
-
Detailed genetic and functional analysis of the hDMDdel52/mdx mouse model.PLoS One. 2020 Dec 23;15(12):e0244215. doi: 10.1371/journal.pone.0244215. eCollection 2020. PLoS One. 2020. PMID: 33362201 Free PMC article.
-
Restoration of Normal NF1 Function with Antisense Morpholino Treatment of Recurrent Pathogenic Patient-Specific Variant c.1466A>G; p.Y489C.J Pers Med. 2021 Dec 7;11(12):1320. doi: 10.3390/jpm11121320. J Pers Med. 2021. PMID: 34945792 Free PMC article.
References
-
- Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Koenig M., Hoffman E.P., Bertelson C.J., Monaco A.P., Feener C., Kunkel L.M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. - PubMed
-
- Flanigan K.M., Dunn D.M., von Niederhausern A., Soltanzadeh P., Gappmaier E., Howard M.T., Sampson J.B., Mendell J.R., Wall C., King W.M., United Dystrophinopathy Project Consortium Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Hum. Mutat. 2009;30:1657–1666. - PMC - PubMed
-
- Ibraghimov-Beskrovnaya O., Ervasti J.M., Leveille C.J., Slaughter C.A., Sernett S.W., Campbell K.P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

