A Unified Walking Model for Dimeric Motor Proteins
- PMID: 30396511
- PMCID: PMC6303276
- DOI: 10.1016/j.bpj.2018.09.032
A Unified Walking Model for Dimeric Motor Proteins
Abstract
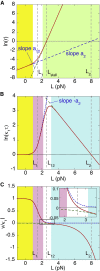
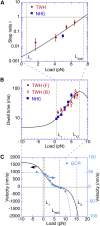
Dimeric motor proteins, kinesin-1, cytoplasmic dynein-1, and myosin-V, move stepwise along microtubules and actin filaments with a regular step size. The motors take backward as well as forward steps. The step ratio r and dwell time τ, which are the ratio of the number of backward steps to the number of forward steps and the time between consecutive steps, respectively, were observed to change with the load. To understand the movement of motor proteins, we constructed a unified and simple mathematical model to explain the load dependencies of r and of τ measured for the above three types of motors quantitatively. Our model consists of three states, and the forward and backward steps are represented by the cycles of transitions visiting different pairs of states among the three, implying that a backward step is not the reversal of a forward step. Each of r and τ is given by a simple expression containing two exponential functions. The experimental data for r and τ for dynein available in the literature are not sufficient for a quantitative analysis, which is in contrast to those for kinesin and myosin-V. We reanalyze the data to obtain r and τ of native dynein to make up the insufficient data to fit them to the model. Our model successfully describes the behavior of r and τ for all of the motors in a wide range of loads from large assisting loads to superstall loads.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Dynamics of dimeric kinesins: Limping, effect of longitudinal force, effects of neck linker extension and mutation, and comparison between kinesin-1 and kinesin-2.Int J Biol Macromol. 2017 Dec;105(Pt 1):1126-1137. doi: 10.1016/j.ijbiomac.2017.07.147. Epub 2017 Jul 25. Int J Biol Macromol. 2017. PMID: 28754624
-
Critical motor number for fractional steps of cytoskeletal filaments in gliding assays.PLoS One. 2012;7(8):e43219. doi: 10.1371/journal.pone.0043219. Epub 2012 Aug 21. PLoS One. 2012. PMID: 22927953 Free PMC article.
-
Force-dependent stepping kinetics of myosin-V.Biophys J. 2005 Jun;88(6):4402-10. doi: 10.1529/biophysj.104.053504. Epub 2005 Mar 11. Biophys J. 2005. PMID: 15764664 Free PMC article.
-
Molecular motors: thermodynamics and the random walk.Proc Biol Sci. 2001 Oct 22;268(1481):2113-22. doi: 10.1098/rspb.2001.1764. Proc Biol Sci. 2001. PMID: 11600075 Free PMC article. Review.
-
Myosin-V, a versatile motor for short-range vesicle transport.Traffic. 2002 Dec;3(12):859-65. doi: 10.1034/j.1600-0854.2002.31202.x. Traffic. 2002. PMID: 12453149 Review.
Cited by
-
Modeling the motion of disease-associated KIF1A heterodimers.Biophys J. 2023 Nov 21;122(22):4348-4359. doi: 10.1016/j.bpj.2023.10.014. Epub 2023 Oct 17. Biophys J. 2023. PMID: 37853694 Free PMC article.
-
Extreme-value analysis in nano-biological systems: applications and implications.Biophys Rev. 2024 Oct 2;16(5):571-579. doi: 10.1007/s12551-024-01239-w. eCollection 2024 Oct. Biophys Rev. 2024. PMID: 39618783 Free PMC article. Review.
-
A model for the chemomechanical coupling of the mammalian cytoplasmic dynein molecular motor.Eur Biophys J. 2019 Oct;48(7):609-619. doi: 10.1007/s00249-019-01386-z. Epub 2019 Jul 5. Eur Biophys J. 2019. PMID: 31278451
-
Dynamics of ATP-dependent and ATP-independent steppings of myosin-V on actin: catch-bond characteristics.J R Soc Interface. 2020 Apr;17(165):20200029. doi: 10.1098/rsif.2020.0029. Epub 2020 Apr 8. J R Soc Interface. 2020. PMID: 32259459 Free PMC article.
-
A model of processive walking and slipping of kinesin-8 molecular motors.Sci Rep. 2021 Apr 13;11(1):8081. doi: 10.1038/s41598-021-87532-0. Sci Rep. 2021. PMID: 33850247 Free PMC article.
References
-
- Howard J. Sinauer Assoc.; Sunderland, MA: 2001. Mechanics of Motor Proteins and the Cytoskeleton.
-
- Schliwa M., editor. Molecular Motors. Wiley-VCH; Weinheim, Germany: 2003.
-
- Jülicher F., Ajdari A., Prost J. Modeling molecular motors. Rev. Mod. Phys. 1997;69:1269–1281.
-
- Astumian R.D. Thermodynamics and kinetics of a Brownian motor. Science. 1997;276:917–922. - PubMed
-
- Kolomeisky A.B., Fisher M.E. Molecular motors: a theorist’s perspective. Annu. Rev. Phys. Chem. 2007;58:675–695. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

