Rickettsia rickettsii Whole-Cell Antigens Offer Protection against Rocky Mountain Spotted Fever in the Canine Host
- PMID: 30396898
- PMCID: PMC6346123
- DOI: 10.1128/IAI.00628-18
Rickettsia rickettsii Whole-Cell Antigens Offer Protection against Rocky Mountain Spotted Fever in the Canine Host
Abstract
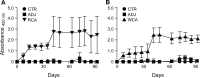
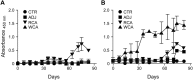
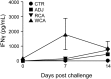
Rocky Mountain spotted fever (RMSF) is a potentially fatal tick-borne disease in people and dogs. RMSF is reported in the United States and several countries in North, Central, and South America. The causative agent of this disease, Rickettsia rickettsii, is transmitted by several species of ticks, including Dermacentor andersoni, Rhipicephalus sanguineus, and Amblyomma americanum RMSF clinical signs generally include fever, headache, nausea, vomiting, muscle pain, lack of appetite, and rash. If untreated, it can quickly progress into a life-threatening illness in people and dogs, with high fatality rates ranging from 30 to 80%. While RMSF has been known for over a century, recent epidemiological data suggest that the numbers of documented cases and the fatality rates remain high in people, particularly during the last two decades in parts of North America. Currently, there are no vaccines available to prevent RMSF in either dogs or people. In this study, we investigated the efficacies of two experimental vaccines, a subunit vaccine containing two recombinant outer membrane proteins as recombinant antigens (RCA) and a whole-cell inactivated antigen vaccine (WCA), in conferring protection against virulent R. rickettsii infection challenge in a newly established canine model for RMSF. Dogs vaccinated with WCA were protected from RMSF, whereas those receiving RCA developed disease similar to that of nonvaccinated R. rickettsii-infected dogs. WCA also reduced the pathogen loads to nearly undetected levels in the blood, lungs, liver, spleen, and brain and induced bacterial antigen-specific immune responses. This study provides the first evidence of the protective ability of WCA against RMSF in dogs.
Keywords: RMSF; Rickettsia; Rocky Mountain spotted fever; tick-borne pathogens; vaccines; vector-borne diseases.
Copyright © 2019 Alhassan et al.
Figures



References
-
- Tribaldos M, Zaldivar Y, Bermudez S, Samudio F, Mendoza Y, Martinez AA, Villalobos R, Eremeeva ME, Paddock CD, Page K, Smith RE, Pascale JM. 2011. Rocky Mountain spotted fever in Panama: a cluster description. J Infect Dev Ctries 5:737–741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

