Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma
- PMID: 30396908
- PMCID: PMC7814996
- DOI: 10.1158/2326-6066.CIR-18-0307
Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma
Abstract
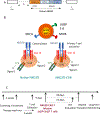
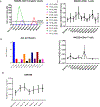
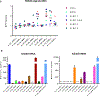
NKG2D ligands are widely expressed in solid and hematologic malignancies but absent or poorly expressed on healthy tissues. We conducted a phase I dose-escalation study to evaluate the safety and feasibility of a single infusion of NKG2D-chimeric antigen receptor (CAR) T cells, without lymphodepleting conditioning in subjects with acute myeloid leukemia/myelodysplastic syndrome or relapsed/refractory multiple myeloma. Autologous T cells were transfected with a γ-retroviral vector encoding a CAR fusing human NKG2D with the CD3ζ signaling domain. Four dose levels (1 × 106-3 × 107 total viable T cells) were evaluated. Twelve subjects were infused [7 acute myeloid leukemia (AML) and 5 multiple myeloma]. NKG2D-CAR products demonstrated a median 75% vector-driven NKG2D expression on CD3+ T cells. No dose-limiting toxicities, cytokine release syndrome, or CAR T cell-related neurotoxicity was observed. No significant autoimmune reactions were noted, and none of the ≥ grade 3 adverse events were attributable to NKG2D-CAR T cells. At the single injection of low cell doses used in this trial, no objective tumor responses were observed. However, hematologic parameters transiently improved in one subject with AML at the highest dose, and cases of disease stability without further therapy or on subsequent treatments were noted. At 24 hours, the cytokine RANTES increased a median of 1.9-fold among all subjects and 5.8-fold among six AML patients. Consistent with preclinical studies, NKG2D-CAR T cell-expansion and persistence were limited. Manufactured NKG2D-CAR T cells exhibited functional activity against autologous tumor cells in vitro, but modifications to enhance CAR T-cell expansion and target density may be needed to boost clinical activity.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Kochenderfer JN, Somerville RPT, Lu T, Yang JC, Sherry RM, Feldman SA, et al. Long-Duration Complete Remissions of Diffuse Large B Cell Lymphoma after Anti-CD19 Chimeric Antigen Receptor T Cell Therapy. Molecular therapy : the journal of the American Society of Gene Therapy 2017;25(10):2245–53 doi 10.1016/j.ymthe.2017.07.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

