Myeloablative vs reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chronic myeloid leukemia
- PMID: 30396912
- PMCID: PMC6234373
- DOI: 10.1182/bloodadvances.2018024844
Myeloablative vs reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chronic myeloid leukemia
Abstract
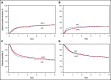
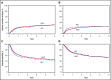
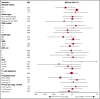
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment of chronic myeloid leukemia (CML). Optimal conditioning intensity for allo-HCT for CML in the era of tyrosine kinase inhibitors (TKIs) is unknown. Using the Center for International Blood and Marrow Transplant Research database, we sought to determine whether reduced-intensity/nonmyeloablative conditioning (RIC) allo-HCT and myeloablative conditioning (MAC) result in similar outcomes in CML patients. We evaluated 1395 CML allo-HCT recipients between the ages of 18 and 60 years. The disease status at transplant was divided into the following categories: chronic phase 1, chronic phase 2 or greater, and accelerated phase. Patients in blast phase at transplant and alternative donor transplants were excluded. The primary outcome was overall survival (OS) after allo-HCT. MAC (n = 1204) and RIC allo-HCT recipients (n = 191) from 2007 to 2014 were included. Patient, disease, and transplantation characteristics were similar, with a few exceptions. Multivariable analysis showed no significant difference in OS between MAC and RIC groups. In addition, leukemia-free survival and nonrelapse mortality did not differ significantly between the 2 groups. Compared with MAC, the RIC group had a higher risk of early relapse after allo-HCT (hazard ratio [HR], 1.85; P = .001). The cumulative incidence of chronic graft-versus-host disease (cGVHD) was lower with RIC than with MAC (HR, 0.77; P = .02). RIC provides similar survival and lower cGVHD compared with MAC and therefore may be a reasonable alternative to MAC for CML patients in the TKI era.
Conflict of interest statement
Conflict-of-interest disclosure: A.A. has advisory board engagements with Boston Biomedical and Incyte Corporation. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Measurable residual disease, conditioning regimen intensity, and age predict outcome of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first remission: A registry analysis of 2292 patients by the Acute Leukemia Working Party European Society of Blood and Marrow Transplantation.Am J Hematol. 2018 Sep;93(9):1142-1152. doi: 10.1002/ajh.25211. Epub 2018 Aug 15. Am J Hematol. 2018. PMID: 29981272
-
Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic hematopoietic stem cell transplantation for acute leukemia patients: a single center experience.Transfus Apher Sci. 2013 Dec;49(3):590-9. doi: 10.1016/j.transci.2013.07.030. Epub 2013 Aug 8. Transfus Apher Sci. 2013. PMID: 23981652
-
The Dilemma of Conditioning Intensity: When Does Myeloablative Conditioning Improve Outcomes for Allogeneic Hematopoietic Cell Transplantation.Biol Blood Marrow Transplant. 2019 Mar;25(3):606-612. doi: 10.1016/j.bbmt.2018.09.012. Epub 2018 Sep 19. Biol Blood Marrow Transplant. 2019. PMID: 30244109
-
Comparison of reduced-intensity and myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and acute lymphoblastic leukemia: a meta-analysis.Stem Cells Dev. 2014 Nov 1;23(21):2535-52. doi: 10.1089/scd.2014.0123. Epub 2014 Sep 17. Stem Cells Dev. 2014. PMID: 25072307 Free PMC article. Review.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
Cited by
-
Transplantation in CML in the TKI era: who, when, and how?Hematology Am Soc Hematol Educ Program. 2022 Dec 9;2022(1):114-122. doi: 10.1182/hematology.2022000329. Hematology Am Soc Hematol Educ Program. 2022. PMID: 36485123 Free PMC article.
-
Allogeneic stem cell transplantation for chronic myeloid leukemia in the TKI era: population-based data from the Swedish CML registry.Bone Marrow Transplant. 2019 Nov;54(11):1764-1774. doi: 10.1038/s41409-019-0513-5. Epub 2019 Apr 8. Bone Marrow Transplant. 2019. PMID: 30962502 Clinical Trial.
-
Watching the Barn Door Open-Timing CML Stem Cell Allografts.Am J Hematol. 2025 Aug;100(8):1268-1270. doi: 10.1002/ajh.27722. Epub 2025 May 20. Am J Hematol. 2025. PMID: 40391878 Free PMC article. No abstract available.
-
Questions concerning tyrosine kinase-inhibitor therapy and transplants in chronic phase chronic myeloid leukaemia.Leukemia. 2022 May;36(5):1227-1236. doi: 10.1038/s41375-022-01522-3. Epub 2022 Mar 25. Leukemia. 2022. PMID: 35338251 Free PMC article. Review.
-
Allogeneic hematopoietic cell transplantation in patients with chronic phase chronic myeloid leukemia in the era of third generation tyrosine kinase inhibitors: A retrospective study by the chronic malignancies working party of the EBMT.Am J Hematol. 2023 Jan;98(1):112-121. doi: 10.1002/ajh.26764. Epub 2022 Oct 31. Am J Hematol. 2023. PMID: 36266607 Free PMC article.
References
-
- Saussele S, Lauseker M, Gratwohl A, et al. ; German CML Study Group. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115(10):1880-1885. - PubMed
-
- Giralt SA, Arora M, Goldman JM, et al. ; Chronic Leukemia Working Committee, Center for International Blood and Marrow Transplant Research. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol. 2007;137(5):461-467. - PubMed
-
- Clift RA, Buckner CD, Thomas ED, et al. . Marrow transplantation for patients in accelerated phase of chronic myeloid leukemia. Blood. 1994;84(12):4368-4373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

