Single-Center Evaluation of the Pharmacokinetics of WCK 5222 (Cefepime-Zidebactam Combination) in Subjects with Renal Impairment
- PMID: 30397067
- PMCID: PMC6325229
- DOI: 10.1128/AAC.01484-18
Single-Center Evaluation of the Pharmacokinetics of WCK 5222 (Cefepime-Zidebactam Combination) in Subjects with Renal Impairment
Abstract
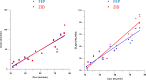
WCK 5222 is a novel β-lactam-β-lactam-enhancer combination of cefepime (FEP) and zidebactam (ZID). ZID is a novel β-lactam enhancer with a dual action of binding to Gram-negative penicillin-binding protein 2 (PBP2) and β-lactamase inhibition. WCK 5222 is being developed as a new therapeutic option for the treatment of complicated multidrug-resistant Gram-negative pathogen infections. We investigated the effect of renal impairment on the pharmacokinetics (PK) and safety of WCK 5222 in 48 subjects based on Cockcroft-Gault-estimated creatinine clearance (CLCR). We enrolled mild (n = 6; CLCR, 60 to <90 ml/min), moderate (n = 6; CLCR, 30 to <60 ml/min), and severe (n = 6; CLCR, <30 ml/min; not on dialysis) impairment, end-stage renal disease (ESRD) on hemodialysis (HD) (n = 6), and matched normal controls (n = 24; CLCR, ≥90 ml/min). Healthy control subjects and mild and moderate renal impairment subjects received a single 60-min intravenous (i.v.) infusion of 3 g WCK 5222 (2 g FEP/1 g ZID); severe renal impairment and HD subjects received a single 60-min i.v. infusion of 1.5 g WCK 5222 (1 g FEP plus 0.5 g ZID). Body and renal clearance decreased, and plasma half-life (t1/2) and the area under the concentration-time curve from time zero extrapolated to infinity (AUC0-∞ [h µg/ml]) increased in a graded relationship with severity of renal impairment for both FEP and ZID. Our findings suggest that dose adjustments for WCK 5222 will be required according to the degree of renal impairment. Overall, WCK 5222 (FEP-ZID) was found to be safe and well tolerated in subjects with normal and impaired renal function. (This study has been registered at ClinicalTrials.gov under identifier NCT02942810.).
Keywords: Acinetobacter baumannii; Enterobacteriaceae; Gram-negative bacterial infections; Pseudomonas aeruginosa; antibacterial agents; bacterial drug resistance; beta-lactamases; chronic kidney disease; drug resistance; multiple drug resistance; pharmacokinetics; pharmacology.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
Single-Center Investigation of the Pharmacokinetics of WCK 4282 (Cefepime-Tazobactam Combination) in Renal Impairment.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e00873-19. doi: 10.1128/AAC.00873-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31332068 Free PMC article. Clinical Trial.
-
Molecular Characterization of WCK 5222 (Cefepime/Zidebactam)-Resistant Mutants Developed from a Carbapenem-Resistant Pseudomonas aeruginosa Clinical Isolate.Microbiol Spectr. 2022 Feb 23;10(1):e0267821. doi: 10.1128/spectrum.02678-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196805 Free PMC article.
-
Potent β-Lactam Enhancer Activity of Zidebactam and WCK 5153 against Acinetobacter baumannii, Including Carbapenemase-Producing Clinical Isolates.Antimicrob Agents Chemother. 2017 Oct 24;61(11):e01238-17. doi: 10.1128/AAC.01238-17. Print 2017 Nov. Antimicrob Agents Chemother. 2017. PMID: 28848013 Free PMC article.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
-
New Drugs for Multidrug-Resistant Gram-Negative Organisms: Time for Stewardship.Drugs. 2019 May;79(7):705-714. doi: 10.1007/s40265-019-01112-1. Drugs. 2019. PMID: 30972660 Review.
Cited by
-
β-Lactam Antibiotics and β-Lactamase Enzymes Inhibitors, Part 2: Our Limited Resources.Pharmaceuticals (Basel). 2022 Apr 13;15(4):476. doi: 10.3390/ph15040476. Pharmaceuticals (Basel). 2022. PMID: 35455473 Free PMC article. Review.
-
Novel Antimicrobial Agents for Gram-Negative Pathogens.Antibiotics (Basel). 2023 Apr 16;12(4):761. doi: 10.3390/antibiotics12040761. Antibiotics (Basel). 2023. PMID: 37107124 Free PMC article. Review.
-
Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions.Eur J Clin Microbiol Infect Dis. 2021 Oct;40(10):2053-2068. doi: 10.1007/s10096-021-04296-1. Epub 2021 Jun 24. Eur J Clin Microbiol Infect Dis. 2021. PMID: 34169446 Free PMC article. Review.
-
What are the optimal pharmacokinetic/pharmacodynamic targets for β-lactamase inhibitors? A systematic review.J Antimicrob Chemother. 2024 May 2;79(5):946-958. doi: 10.1093/jac/dkae058. J Antimicrob Chemother. 2024. PMID: 38459763 Free PMC article.
-
Antibiotic resistance breakers: current approaches and future directions.FEMS Microbiol Rev. 2019 Sep 1;43(5):490-516. doi: 10.1093/femsre/fuz014. FEMS Microbiol Rev. 2019. PMID: 31150547 Free PMC article. Review.
References
-
- Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Olivera A. 2017. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “beta-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. - PMC - PubMed
-
- Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Olivera A. 2017. Potent β-lactam enhancer activity of zidebactam and WCK 5153 against Acinetobacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother 61:e01238-17. doi:10.1128/AAC.01238-17. - DOI - PMC - PubMed
-
- Moya B, Barcelo I, Udaykar A, Bhagwat S, Patel M, Bou G, Oliver A. 2016. WCK 5222 [cefepime-zidebactam, FEP-ZID]: mechanistic basis behind novel β-lactam–β-lactam enhancer combination against metallo-β-lactamase (MBL)-producing E. coli (EC), K. pneumoniae (KP), P. aeruginosa (PA) and its impact on therapeutically relevant bactericidal exposures assessed through in vitro pharmacodynamic modelling (IVPM) and mouse lung eradication studies, abstr 1982. IDWeek 2016, 26 to 30 October 2016, New Orleans, LA.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical