Inducible Cell Fusion Permits Use of Competitive Fitness Profiling in the Human Pathogenic Fungus Aspergillus fumigatus
- PMID: 30397071
- PMCID: PMC6325235
- DOI: 10.1128/AAC.01615-18
Inducible Cell Fusion Permits Use of Competitive Fitness Profiling in the Human Pathogenic Fungus Aspergillus fumigatus
Erratum in
-
Correction for Macdonald et al., "Inducible Cell Fusion Permits Use of Competitive Fitness Profiling in the Human Pathogenic Fungus Aspergillus fumigatus".Antimicrob Agents Chemother. 2019 May 24;63(6):e00706-19. doi: 10.1128/AAC.00706-19. Print 2019 Jun. Antimicrob Agents Chemother. 2019. PMID: 31126922 Free PMC article. No abstract available.
Abstract
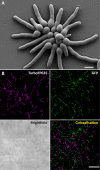
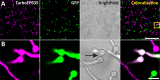
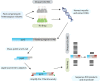
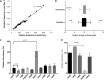
Antifungal agents directed against novel therapeutic targets are required for treating invasive, chronic, and allergic Aspergillus infections. Competitive fitness profiling technologies have been used in a number of bacterial and yeast systems to identify druggable targets; however, the development of similar systems in filamentous fungi is complicated by the fact that they undergo cell fusion and heterokaryosis. Here, we demonstrate that cell fusion in Aspergillus fumigatus under standard culture conditions is not predominately constitutive, as with most ascomycetes, but can be induced by a range of extracellular stressors. Using this knowledge, we have developed a barcode-free genetic profiling system that permits high-throughput parallel determination of strain fitness in a collection of diploid A. fumigatus mutants. We show that heterozygous cyp51A and arf2 null mutants have reduced fitness in the presence of itraconazole and brefeldin A, respectively, and a heterozygous atp17 null mutant is resistant to brefeldin A.
Keywords: Aspergillus fumigatus; anastomosis; antifungal; chemical genomics; functional genomics; hyphal fusion; imaging.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
Multi-azole resistance in Aspergillus fumigatus.Int J Antimicrob Agents. 2006 Nov;28(5):450-3. doi: 10.1016/j.ijantimicag.2006.08.017. Epub 2006 Oct 10. Int J Antimicrob Agents. 2006. PMID: 17034993
-
First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey.J Infect Chemother. 2015 Aug;21(8):581-6. doi: 10.1016/j.jiac.2015.04.012. Epub 2015 May 14. J Infect Chemother. 2015. PMID: 26048062
-
Progressive Dispersion of Azole Resistance in Aspergillus fumigatus: Fatal Invasive Aspergillosis in a Patient with Acute Myeloid Leukemia Infected with an A. fumigatus Strain with a cyp51A TR46 Y121F M172I T289A Allele.Antimicrob Agents Chemother. 2017 Jul 25;61(8):e00270-17. doi: 10.1128/AAC.00270-17. Print 2017 Aug. Antimicrob Agents Chemother. 2017. PMID: 28743702 Free PMC article.
-
Azole resistant Aspergillus fumigatus: an emerging problem.Med Mal Infect. 2013 Apr;43(4):139-45. doi: 10.1016/j.medmal.2013.02.010. Epub 2013 Apr 4. Med Mal Infect. 2013. PMID: 23562488 Review.
-
Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms.Future Microbiol. 2014;9(5):697-711. doi: 10.2217/fmb.14.27. Future Microbiol. 2014. PMID: 24957095 Review.
Cited by
-
Advances in fungal chemical genomics for the discovery of new antifungal agents.Ann N Y Acad Sci. 2021 Jul;1496(1):5-22. doi: 10.1111/nyas.14484. Epub 2020 Aug 28. Ann N Y Acad Sci. 2021. PMID: 32860238 Free PMC article. Review.
-
Recent developments in Aspergillus fumigatus research: diversity, drugs, and disease.Microbiol Mol Biol Rev. 2025 Mar 27;89(1):e0001123. doi: 10.1128/mmbr.00011-23. Epub 2025 Feb 10. Microbiol Mol Biol Rev. 2025. PMID: 39927770 Review.
-
Permissiveness and competition within and between Neurospora crassa syncytia.Genetics. 2023 Aug 9;224(4):iyad112. doi: 10.1093/genetics/iyad112. Genetics. 2023. PMID: 37313736 Free PMC article.
-
Tackling the emerging threat of antifungal resistance to human health.Nat Rev Microbiol. 2022 Sep;20(9):557-571. doi: 10.1038/s41579-022-00720-1. Epub 2022 Mar 29. Nat Rev Microbiol. 2022. PMID: 35352028 Free PMC article. Review.
-
Fungal Pathogens: Shape-Shifting Invaders.Trends Microbiol. 2020 Nov;28(11):922-933. doi: 10.1016/j.tim.2020.05.001. Epub 2020 May 27. Trends Microbiol. 2020. PMID: 32474010 Free PMC article. Review.
References
-
- Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young J-AH, Bennett JE. 2016. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:433–442. doi:10.1093/cid/ciw444. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

