Interleukin-8 Activates Breast Cancer-Associated Adipocytes and Promotes Their Angiogenesis- and Tumorigenesis-Promoting Effects
- PMID: 30397072
- PMCID: PMC6321878
- DOI: 10.1128/MCB.00332-18
Interleukin-8 Activates Breast Cancer-Associated Adipocytes and Promotes Their Angiogenesis- and Tumorigenesis-Promoting Effects
Abstract
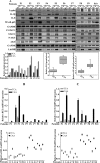
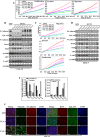
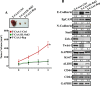
Increasing evidence supports the critical role of active stromal adipocytes in breast cancer development and spread. However, the mediators and the mechanisms of action are still elusive. We show here that cancer-associated adipocytes (CAAs) isolated from 10 invasive breast carcinomas are proinflammatory and exhibit active phenotypes, including higher proliferative, invasive, and migratory capacities compared to their adjacent tumor-counterpart adipocytes (TCAs). Furthermore, all CAAs secreted higher level of interleukin-8 (IL-8), which is critical in mediating the paracrine procarcinogenic effects of these cells. Importantly, ectopic expression of IL-8 in TCA cells activated them and enhanced their procarcinogenic effects both in vitro, in a STAT3-dependent manner, and in vivo In contrast, inhibition of the IL-8 signaling using specific short hairpin RNA, anti-IL-8 antibody, or reparixin suppressed the active features of CAAs, including their non-cell-autonomous tumor-promoting activities both on breast luminal cells and in orthotopic tumor xenografts in mice. IL-8 played also an important role in enhancing the proangiogenic effects of breast adipocytes. These results provide clear indication that IL-8 plays key roles in the activation of breast CAAs and acts as a major mediator for their paracrine protumorigenic effects. Thus, targeting CAAs by inhibiting the IL-8 pathway could have great therapeutic value.
Keywords: IL-8; STAT3; adipocytes; breast cancer; reparixin.
Copyright © 2019 American Society for Microbiology.
Figures







Similar articles
-
Senescent Breast Luminal Cells Promote Carcinogenesis through Interleukin-8-Dependent Activation of Stromal Fibroblasts.Mol Cell Biol. 2019 Jan 3;39(2):e00359-18. doi: 10.1128/MCB.00359-18. Print 2019 Jan 15. Mol Cell Biol. 2019. PMID: 30397077 Free PMC article.
-
Decorin (DCN) Downregulation Activates Breast Stromal Fibroblasts and Promotes Their Pro-Carcinogenic Effects through the IL-6/STAT3/AUF1 Signaling.Cells. 2024 Apr 14;13(8):680. doi: 10.3390/cells13080680. Cells. 2024. PMID: 38667295 Free PMC article.
-
p16INK4A represses breast stromal fibroblasts migration/invasion and their VEGF-A-dependent promotion of angiogenesis through Akt inhibition.Neoplasia. 2012 Dec;14(12):1269-77. doi: 10.1593/neo.121632. Neoplasia. 2012. PMID: 23308058 Free PMC article.
-
Cancer-associated adipocytes: emerging supporters in breast cancer.J Exp Clin Cancer Res. 2020 Aug 12;39(1):156. doi: 10.1186/s13046-020-01666-z. J Exp Clin Cancer Res. 2020. PMID: 32787888 Free PMC article. Review.
-
Cancer-Associated Adipocytes in Breast Cancer: Causes and Consequences.Int J Mol Sci. 2021 Apr 6;22(7):3775. doi: 10.3390/ijms22073775. Int J Mol Sci. 2021. PMID: 33917351 Free PMC article. Review.
Cited by
-
Nutrients Lowering Obesity-Linked Chemokines Blamable for Metastasis.Int J Mol Sci. 2025 Mar 4;26(5):2275. doi: 10.3390/ijms26052275. Int J Mol Sci. 2025. PMID: 40076892 Free PMC article. Review.
-
Cancer Stem Cells: An Ever-Hiding Foe.Exp Suppl. 2022;113:219-251. doi: 10.1007/978-3-030-91311-3_8. Exp Suppl. 2022. PMID: 35165866
-
Adipocytes and microRNAs Crosstalk: A Key Tile in the Mosaic of Breast Cancer Microenvironment.Cancers (Basel). 2019 Sep 27;11(10):1451. doi: 10.3390/cancers11101451. Cancers (Basel). 2019. PMID: 31569710 Free PMC article. Review.
-
Adipose Tissue Properties in Tumor-Bearing Breasts.Front Oncol. 2020 Aug 21;10:1506. doi: 10.3389/fonc.2020.01506. eCollection 2020. Front Oncol. 2020. PMID: 32974182 Free PMC article.
-
The Adipocyte-Macrophage Relationship in Cancer: A Potential Target for Antioxidant Therapy.Antioxidants (Basel). 2023 Jan 4;12(1):126. doi: 10.3390/antiox12010126. Antioxidants (Basel). 2023. PMID: 36670988 Free PMC article. Review.
References
-
- Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. 2011. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 71:2455–2465. doi:10.1158/0008-5472.CAN-10-3323. - DOI - PubMed
-
- Wang YY, Attane C, Milhas D, Dirat B, Dauvillier S, Guerard A, Gilhodes J, Lazar I, Alet N, Laurent V, Le Gonidec S, Biard D, Herve C, Bost F, Ren GS, Bono F, Escourrou G, Prentki M, Nieto L, Valet P, Muller C. 2017. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2:e87489. doi:10.1172/jci.insight.87489. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
