Hypoxia Restrains Lipid Utilization via Protein Kinase A and Adipose Triglyceride Lipase Downregulation through Hypoxia-Inducible Factor
- PMID: 30397073
- PMCID: PMC6321877
- DOI: 10.1128/MCB.00390-18
Hypoxia Restrains Lipid Utilization via Protein Kinase A and Adipose Triglyceride Lipase Downregulation through Hypoxia-Inducible Factor
Abstract
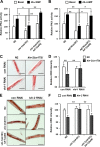
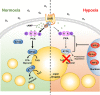
Oxygen is a key molecule for efficient energy production in living organisms. Although aerobic organisms have adaptive processes to survive in low-oxygen environments, it is poorly understood how lipolysis, the first step of energy production from stored lipid metabolites, would be modulated during hypoxia. Here, we demonstrate that fasting-induced lipolysis is downregulated by hypoxia through the hypoxia-inducible factor (HIF) signaling pathway. In Caenorhabditis elegans and mammalian adipocytes, hypoxia suppressed protein kinase A (PKA)-stimulated lipolysis, which is evolutionarily well conserved. During hypoxia, the levels of PKA activity and adipose triglyceride lipase (ATGL) protein were downregulated, resulting in attenuated fasting-induced lipolysis. In worms, HIF stabilization was sufficient to moderate the suppressive effect of hypoxia on lipolysis through ATGL and PKA inhibition. These data suggest that HIF activation under hypoxia plays key roles in the suppression of lipolysis, which might preserve energy resources in both C. elegans and mammalian adipocytes.
Keywords: ATGL; HIF; PKA; hypoxia; lipolysis.
Copyright © 2019 American Society for Microbiology.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
