Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration
- PMID: 30397118
- PMCID: PMC6255167
- DOI: 10.1073/pnas.1802519115
Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration
Abstract
Recessive Stargardt disease (STGD1) is an inherited blinding disorder caused by mutations in the Abca4 gene. ABCA4 is a flippase in photoreceptor outer segments (OS) that translocates retinaldehyde conjugated to phosphatidylethanolamine across OS disc membranes. Loss of ABCA4 in Abca4-/- mice and STGD1 patients causes buildup of lipofuscin in the retinal pigment epithelium (RPE) and degeneration of photoreceptors, leading to blindness. No effective treatment currently exists for STGD1. Here we show by several approaches that ABCA4 is additionally expressed in RPE cells. (i) By in situ hybridization analysis and by RNA-sequencing analysis, we show the Abca4 mRNA is expressed in human and mouse RPE cells. (ii) By quantitative immunoblotting, we show that the level of ABCA4 protein in homogenates of wild-type mouse RPE is about 1% of the level in neural retina homogenates. (iii) ABCA4 immunofluorescence is present in RPE cells of wild-type and Mertk-/- but not Abca4-/- mouse retina sections, where it colocalizes with endolysosomal proteins. To elucidate the role of ABCA4 in RPE cells, we generated a line of genetically modified mice that express ABCA4 in RPE cells but not in photoreceptors. Mice from this line on the Abca4-/- background showed partial rescue of photoreceptor degeneration and decreased lipofuscin accumulation compared with nontransgenic Abca4-/- mice. We propose that ABCA4 functions to recycle retinaldehyde released during proteolysis of rhodopsin in RPE endolysosomes following daily phagocytosis of distal photoreceptor OS. ABCA4 deficiency in the RPE may play a role in the pathogenesis of STGD1.
Keywords: ABCA4; Stargardt disease; bisretinoid; lipofuscin; retinal pigment epithelium.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

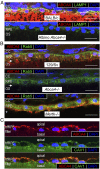
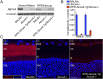
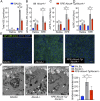

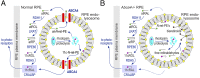
References
-
- Wald G. Molecular basis of visual excitation. Science. 1968;162:230–239. - PubMed
-
- Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275:11034–11043. - PubMed
-
- Sun H, Nathans J. Stargardt’s ABCR is localized to the disc membrane of retinal rod outer segments. Nat Genet. 1997;17:15–16. - PubMed
-
- Allikmets R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;17:122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

