Human-like hyperplastic prostate with low ZIP1 induced solely by Zn deficiency in rats
- PMID: 30397150
- PMCID: PMC6255182
- DOI: 10.1073/pnas.1813956115
Human-like hyperplastic prostate with low ZIP1 induced solely by Zn deficiency in rats
Abstract
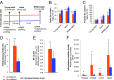
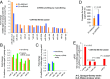
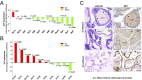
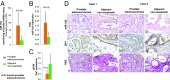
Prostate cancer is a leading cause of cancer death in men over 50 years of age, and there is a characteristic marked decrease in Zn content in the malignant prostate cells. The cause and consequences of this loss have thus far been unknown. We found that in middle-aged rats a Zn-deficient diet reduces prostatic Zn levels (P = 0.025), increases cellular proliferation, and induces an inflammatory phenotype with COX-2 overexpression. This hyperplastic/inflammatory prostate has a human prostate cancer-like microRNA profile, with up-regulation of the Zn-homeostasis-regulating miR-183-96-182 cluster (fold change = 1.41-2.38; P = 0.029-0.0003) and down-regulation of the Zn importer ZIP1 (target of miR-182), leading to a reduction of prostatic Zn. This inverse relationship between miR-182 and ZIP1 also occurs in human prostate cancer tissue, which is known for Zn loss. The discovery that the Zn-depleted middle-aged rat prostate has a metabolic phenotype resembling that of human prostate cancer, with a 10-fold down-regulation of citric acid (P = 0.0003), links citrate reduction directly to prostatic Zn loss, providing the underlying mechanism linking dietary Zn deficiency with miR-183-96-182 overexpression, ZIP1 down-regulation, prostatic Zn loss, and the resultant citrate down-regulation, changes mimicking features of human prostate cancer. Thus, dietary Zn deficiency during rat middle age produces changes that mimic those of human prostate carcinoma and may increase the risk for prostate cancer, supporting the need for assessment of Zn supplementation in its prevention.
Keywords: dietary Zn intake; prostate cancer metabolic phenotype; prostate cancer risk; untargeted metabolomics profiling; untargeted miRNA profiling.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yatani R, et al. Geographic pathology of latent prostatic carcinoma. Int J Cancer. 1982;29:611–616. - PubMed
-
- Mawson CA, Fischer MI. The occurrence of zinc in the human prostate gland. Can J Med Sci. 1952;30:336–339. - PubMed
-
- Zaichick VYe, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: Normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29:565–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

