Autocrine signaling by an Aplysia neurotrophin forms a presynaptic positive feedback loop
- PMID: 30397154
- PMCID: PMC6255211
- DOI: 10.1073/pnas.1810649115
Autocrine signaling by an Aplysia neurotrophin forms a presynaptic positive feedback loop
Abstract
Whereas short-term plasticity is often initiated on one side of the synapse, long-term plasticity involves coordinated changes on both sides, implying extracellular signaling. We have investigated the possible signaling role of an Aplysia neurotrophin (ApNT) in facilitation induced by serotonin (5HT) at sensory-to-motor neuron synapses in culture. ApNT is an ortholog of mammalian BDNF, which has been reported to act as either an anterograde, retrograde, or autocrine signal, so that its pre- and postsynaptic sources and targets remain unclear. We now report that ApNT acts as a presynaptic autocrine signal that forms part of a positive feedback loop with ApTrk and PKA. That loop stimulates spontaneous transmitter release, which recruits postsynaptic mechanisms, and presynaptic protein synthesis during the transition from short- to intermediate-term facilitation and may also initiate gene regulation to trigger the transition to long-term facilitation. These results suggest that a presynaptic ApNT feedback loop plays several key roles during consolidation of learning-related synaptic plasticity.
Keywords: Aplysia; autocrine; facilitation; neurotrophin; presynaptic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

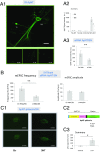
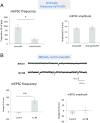
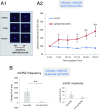
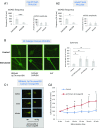

Similar articles
-
Anterograde and retrograde signaling by an Aplysia neurotrophin forms a transsynaptic functional unit.Proc Natl Acad Sci U S A. 2018 Nov 13;115(46):E10951-E10960. doi: 10.1073/pnas.1810650115. Epub 2018 Oct 30. Proc Natl Acad Sci U S A. 2018. PMID: 30377269 Free PMC article.
-
Rapid increase in clusters of synaptophysin at onset of homosynaptic potentiation in Aplysia.Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11656-61. doi: 10.1073/pnas.1102695108. Epub 2011 Jun 27. Proc Natl Acad Sci U S A. 2011. PMID: 21709228 Free PMC article.
-
Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of classical conditioning at Aplysia sensory-motor neuron synapses in isolated cell culture.J Neurosci. 1998 Jan 1;18(1):458-66. doi: 10.1523/JNEUROSCI.18-01-00458.1998. J Neurosci. 1998. PMID: 9412522 Free PMC article.
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
-
New tricks for an old slug: the critical role of postsynaptic mechanisms in learning and memory in Aplysia.Prog Brain Res. 2008;169:277-92. doi: 10.1016/S0079-6123(07)00017-9. Prog Brain Res. 2008. PMID: 18394481 Free PMC article. Review.
Cited by
-
Recent advances in understanding context-dependent mechanisms controlling neurotrophin signaling and function.F1000Res. 2019 Sep 19;8:F1000 Faculty Rev-1658. doi: 10.12688/f1000research.19174.1. eCollection 2019. F1000Res. 2019. PMID: 31583078 Free PMC article. Review.
-
Defective synaptic plasticity in a model of Coffin-Lowry syndrome is rescued by simultaneously targeting PKA and MAPK pathways.Learn Mem. 2022 Nov 29;29(12):435-446. doi: 10.1101/lm.053625.122. Print 2022 Dec. Learn Mem. 2022. PMID: 36446603 Free PMC article.
-
Dynamics and Mechanisms of ERK Activation after Different Protocols that Induce Long-Term Synaptic Facilitation in Aplysia.Oxf Open Neurosci. 2022 Oct 18;2:kvac014. doi: 10.1093/oons/kvac014. eCollection 2023. Oxf Open Neurosci. 2022. PMID: 37649778 Free PMC article.
-
Anterograde and retrograde signaling by an Aplysia neurotrophin forms a transsynaptic functional unit.Proc Natl Acad Sci U S A. 2018 Nov 13;115(46):E10951-E10960. doi: 10.1073/pnas.1810650115. Epub 2018 Oct 30. Proc Natl Acad Sci U S A. 2018. PMID: 30377269 Free PMC article.
-
A kinetic model for Brain-Derived Neurotrophic Factor mediated spike timing-dependent LTP.PLoS Comput Biol. 2019 Apr 24;15(4):e1006975. doi: 10.1371/journal.pcbi.1006975. eCollection 2019 Apr. PLoS Comput Biol. 2019. PMID: 31017891 Free PMC article.
References
-
- Rosenzweig MR, Bennett EL, Colombo PJ, Lee DW, Serrano PA. Short-term, intermediate-term, and long-term memories. Behav Brain Res. 1993;57:193–198. - PubMed
-
- Raymond CR. LTP forms 1, 2 and 3: Different mechanisms for the “long” in long-term potentiation. Trends Neurosci. 2007;30:167–175. - PubMed
-
- Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. - PubMed
-
- Hawkins RD, Mayford MR, Kandel ER. A comparative analysis of the molecular mechanisms contributing to implicit and explicit memory storage in Aplysia and in the hippocampus. In: Byrne JH, editor. Learning and Memory: A Comprehensive Reference. 2nd Ed. Elsevier; Oxford: 2016. pp. 5–32.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

