Differential lateral and basal tension drive folding of Drosophila wing discs through two distinct mechanisms
- PMID: 30397306
- PMCID: PMC6218478
- DOI: 10.1038/s41467-018-06497-3
Differential lateral and basal tension drive folding of Drosophila wing discs through two distinct mechanisms
Abstract
Epithelial folding transforms simple sheets of cells into complex three-dimensional tissues and organs during animal development. Epithelial folding has mainly been attributed to mechanical forces generated by an apically localized actomyosin network, however, contributions of forces generated at basal and lateral cell surfaces remain largely unknown. Here we show that a local decrease of basal tension and an increased lateral tension, but not apical constriction, drive the formation of two neighboring folds in developing Drosophila wing imaginal discs. Spatially defined reduction of extracellular matrix density results in local decrease of basal tension in the first fold; fluctuations in F-actin lead to increased lateral tension in the second fold. Simulations using a 3D vertex model show that the two distinct mechanisms can drive epithelial folding. Our combination of lateral and basal tension measurements with a mechanical tissue model reveals how simple modulations of surface and edge tension drive complex three-dimensional morphological changes.
Conflict of interest statement
The authors declare no competing interests.
Figures
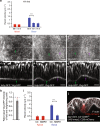
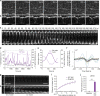
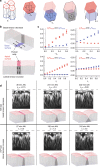
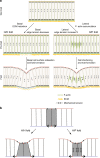
Similar articles
-
Increased lateral tension is sufficient for epithelial folding in Drosophila.Development. 2020 Dec 4;147(23):dev194316. doi: 10.1242/dev.194316. Development. 2020. PMID: 33277300
-
Local weakening of cell-extracellular matrix adhesion triggers basal epithelial tissue folding.EMBO J. 2025 Apr;44(7):2002-2024. doi: 10.1038/s44318-025-00384-6. Epub 2025 Feb 17. EMBO J. 2025. PMID: 39962267 Free PMC article.
-
Wingless counteracts epithelial folding by increasing mechanical tension at basal cell edges in Drosophila.Development. 2020 Mar 11;147(5):dev184713. doi: 10.1242/dev.184713. Development. 2020. PMID: 32161062
-
Forces shaping the Drosophila wing.Mech Dev. 2017 Apr;144(Pt A):23-32. doi: 10.1016/j.mod.2016.10.003. Epub 2016 Oct 23. Mech Dev. 2017. PMID: 27784612 Review.
-
Epithelial polarity and morphogenesis.Curr Opin Cell Biol. 2011 Oct;23(5):540-6. doi: 10.1016/j.ceb.2011.07.005. Epub 2011 Jul 30. Curr Opin Cell Biol. 2011. PMID: 21807488 Review.
Cited by
-
Active morphogenesis of patterned epithelial shells.Elife. 2023 Jan 17;12:e75878. doi: 10.7554/eLife.75878. Elife. 2023. PMID: 36649186 Free PMC article.
-
Buckling of an Epithelium Growing under Spherical Confinement.Dev Cell. 2020 Sep 14;54(5):655-668.e6. doi: 10.1016/j.devcel.2020.07.019. Epub 2020 Aug 14. Dev Cell. 2020. PMID: 32800097 Free PMC article.
-
Basement membrane patterning by spatial deployment of a secretion-regulating protease.bioRxiv [Preprint]. 2024 Jul 10:2024.07.06.602330. doi: 10.1101/2024.07.06.602330. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 May 20;122(20):e2412161122. doi: 10.1073/pnas.2412161122. PMID: 39026720 Free PMC article. Updated. Preprint.
-
Homeotic compartment curvature and tension control spatiotemporal folding dynamics.Nat Commun. 2023 Feb 3;14(1):594. doi: 10.1038/s41467-023-36305-6. Nat Commun. 2023. PMID: 36737611 Free PMC article.
-
Cultivation and Live Imaging of Drosophila Imaginal Discs.Methods Mol Biol. 2022;2540:317-334. doi: 10.1007/978-1-0716-2541-5_16. Methods Mol Biol. 2022. PMID: 35980586
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

