A chemoselective strategy for late-stage functionalization of complex small molecules with polypeptides and proteins
- PMID: 30397320
- PMCID: PMC6454892
- DOI: 10.1038/s41557-018-0154-0
A chemoselective strategy for late-stage functionalization of complex small molecules with polypeptides and proteins
Abstract
Conjugates between proteins and small molecules enable access to a vast chemical space that is not achievable with either type of molecule alone; however, the paucity of specific reactions capable of functionalizing proteins and natural products presents a formidable challenge for preparing conjugates. Here we report a strategy for conjugating electron-rich (hetero)arenes to polypeptides and proteins. Our bioconjugation technique exploits the electrophilic reactivity of an oxidized selenocysteine residue in polypeptides and proteins, and the electron-rich character of certain small molecules to provide bioconjugates in excellent yields under mild conditions. This conjugation chemistry enabled the synthesis of peptide-vancomycin conjugates without the prefunctionalization of vancomycin. These conjugates have an enhanced in vitro potency for resistant Gram-positive and Gram-negative pathogens. Additionally, we show that a 6 kDa affibody protein and a 150 kDa immunoglobulin-G antibody could be modified without diminishing bioactivity.
Conflict of interest statement
Competing interests
K.D.J., Z.S. and O.P. are employees of Visterra Inc. D.T.C., C.Z., S.L.B., and B.L.P. are inventors on a patent filed by MIT-TLO to cover this work (US patent application no. 15187169).
Figures


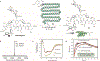
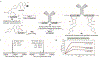

Similar articles
-
Synthesis of Bioactive Complex Small Molecule-Ciprofloxacin Conjugates and Evaluation of Their Antibacterial Activity.ACS Comb Sci. 2020 Sep 14;22(9):440-445. doi: 10.1021/acscombsci.0c00060. Epub 2020 Jul 21. ACS Comb Sci. 2020. PMID: 32691584
-
Electrochemical Modification of Polypeptides at Selenocysteine.Angew Chem Int Ed Engl. 2023 Dec 11;62(50):e202313037. doi: 10.1002/anie.202313037. Epub 2023 Nov 9. Angew Chem Int Ed Engl. 2023. PMID: 37818778
-
Site-Specifically Labeled Immunoconjugates for Molecular Imaging--Part 2: Peptide Tags and Unnatural Amino Acids.Mol Imaging Biol. 2016 Apr;18(2):153-65. doi: 10.1007/s11307-015-0920-y. Mol Imaging Biol. 2016. PMID: 26754791 Free PMC article. Review.
-
More than add-on: chemoselective reactions for the synthesis of functional peptides and proteins.Curr Opin Chem Biol. 2014 Oct;22:62-9. doi: 10.1016/j.cbpa.2014.09.018. Epub 2014 Oct 3. Curr Opin Chem Biol. 2014. PMID: 25285752 Review.
-
Stable and Potent Selenomab-Drug Conjugates.Cell Chem Biol. 2017 Apr 20;24(4):433-442.e6. doi: 10.1016/j.chembiol.2017.02.012. Epub 2017 Mar 16. Cell Chem Biol. 2017. PMID: 28330604 Free PMC article.
Cited by
-
A selenoxide for single-atom protein modification of tyrosine residues enabled by water-resistant chalcogen and hydrogen bonding.Nat Chem. 2025 Jun 4. doi: 10.1038/s41557-025-01842-8. Online ahead of print. Nat Chem. 2025. PMID: 40467891
-
Site-selective photocatalytic functionalization of peptides and proteins at selenocysteine.Nat Commun. 2022 Nov 12;13(1):6885. doi: 10.1038/s41467-022-34530-z. Nat Commun. 2022. PMID: 36371402 Free PMC article.
-
All-in-one disulfide bridging enables the generation of antibody conjugates with modular cargo loading.Chem Sci. 2022 Jul 20;13(30):8781-8790. doi: 10.1039/d2sc02198f. eCollection 2022 Aug 4. Chem Sci. 2022. PMID: 35975158 Free PMC article.
-
Electrochemical Late-Stage Functionalization.Chem Rev. 2023 Oct 11;123(19):11269-11335. doi: 10.1021/acs.chemrev.3c00158. Epub 2023 Sep 26. Chem Rev. 2023. PMID: 37751573 Free PMC article. Review.
-
Biological, chemical, and biochemical strategies for modifying glycopeptide antibiotics.J Biol Chem. 2019 Dec 6;294(49):18769-18783. doi: 10.1074/jbc.REV119.006349. Epub 2019 Oct 31. J Biol Chem. 2019. PMID: 31672921 Free PMC article. Review.
References
-
- Drake PM & Rabuka D An emerging playbook for antibody–drug conjugates: lessons from the laboratory and clinic suggest a strategy for improving efficacy and safety. Curr. Opin. Chem. Biol 28, 174–180 (2015). - PubMed
-
- Ueda T Next-generation optimized biotherapeutics — A review and preclinical study. Biochim. Biophys. Acta BBA - Proteins Proteomics 1844, 2053–2057 (2014). - PubMed
-
- Hamann PR et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 13, 47–58 (2002). - PubMed
-
- Lewis Phillips GD et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

