Catalytic activation of unstrained C(aryl)-C(aryl) bonds in 2,2'-biphenols
- PMID: 30397321
- PMCID: PMC6383370
- DOI: 10.1038/s41557-018-0157-x
Catalytic activation of unstrained C(aryl)-C(aryl) bonds in 2,2'-biphenols
Abstract
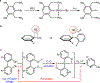
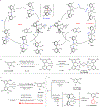
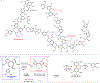
Transition metal catalysis has emerged as an important means for C-C activation that allows mild and selective transformations. However, the current scope of C-C bonds that can be activated is primarily restricted to either highly strained systems or more polarized C-C bonds. In contrast, the catalytic activation of non-polar and unstrained C-C moieties remains an unmet challenge. Here we report a general approach for the catalytic activation of the unstrained C(aryl)-C(aryl) bonds in 2,2'-biphenols. The key is to utilize the phenol moiety as a handle to install phosphinites as a recyclable directing group. Using hydrogen gas as the reductant, monophenols are obtained with a low catalyst loading and high functional group tolerance. This approach is also applied to the synthesis of 2,3,4-trisubstituted phenols. A further mechanistic study suggests that the C-C activation step is mediated by a rhodium(I) monohydride species. Finally, a preliminary study on breaking the inert biphenolic moieties in lignin models is illustrated.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

References
-
- Drahl MA, Manpadi M & Williams LJ C−C fragmentation: origins and recent applications. Angew. Chem. Int. Ed. 52, 11222–11251 (2013). - PubMed
-
- Fahim MA, Alsahhaf TA & Elkilani A Fluidised catalytic cracking, in Fundamentals of Petroleum Refining. 199–235 (Elsevier, 2010).
-
- Murakami M & Ito Y Cleavage of carbon−carbon single bonds by transition metals. Top. Organomet. Chem. 3, 97–129 (1999).
-
- Rybtchinski B & Milstein D Metal insertion into C−C bonds in solution. Angew. Chem. Int. Ed. 38, 870–883 (1999). - PubMed
-
- Chen F, Wang T & Jiao N Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev. 114, 8613–8661 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

