Efficacy and safety of autologous stem cell transplantation for decompensated liver cirrhosis: A retrospective cohort study
- PMID: 30397424
- PMCID: PMC6212545
- DOI: 10.4252/wjsc.v10.i10.138
Efficacy and safety of autologous stem cell transplantation for decompensated liver cirrhosis: A retrospective cohort study
Abstract
Aim: To evaluate the long-term efficacy and safety of autologous stem cell transplantation (SCT) for decompensated liver cirrhosis.
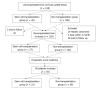
Methods: Consecutive patients with decompensated liver cirrhosis were included and assigned into the SCT group and non-transplantation (non-SCT) group according to whether they received SCT treatment. Patients were followed up for ten years. The long-term survival rate and incidence of hepatocellular carcinoma (HCC) were compared between groups.
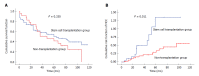
Results: A total of 159 patients were enrolled, including 27 cases in the SCT group and 132 cases in the non-SCT group. The baseline characteristics were significantly different between the two groups. Propensity score matching (PSM) was used to match SCT and non-SCT patients. After PSM, 92 subjects were enrolled in the final analysis, including 23 cases in the SCT group and 69 cases in the non-SCT group. The overall mortality was 73.9% and 55.1%, and the median survival period was 48 and 64 mo, respectively. However, no significant difference was found in the long-term survival rate between the two groups (P > 0.05). In addition, the incidence of HCC was higher in the SCT group than in the non-SCT group (47.8% vs 21.7%, P < 0.05). After adjusting for other covariates, SCT (OR = 3.065, 95%CI: 1.378-6.814) and age (OR = 1.061, 95%CI: 1.021-1.102) were independently correlated with the development of HCC in this decompensated liver cirrhosis cohort.
Conclusion: Autologous SCT may fail to improve the long-term efficacy and increase the incidence of HCC for decompensated liver cirrhosis. Close monitoring of HCC is strongly recommended in patients undergoing autologous SCT.
Keywords: Decompensated liver cirrhosis; Hepatocellular carcinoma; Propensity score matching; Stem cell transplantation.
Conflict of interest statement
Conflict-of-interest statement: All authors declare no potential conflict of interest.
Figures
Similar articles
-
Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Transplantation for Patients with Decompensated Liver Cirrhosis.J Gastrointest Surg. 2023 May;27(5):926-931. doi: 10.1007/s11605-022-05528-1. Epub 2023 Jan 26. J Gastrointest Surg. 2023. PMID: 36703021 Free PMC article. Clinical Trial.
-
Long-term Outcomes of Autologous Peripheral Blood Stem Cell Transplantation in Patients With Cirrhosis.Clin Gastroenterol Hepatol. 2019 May;17(6):1175-1182.e2. doi: 10.1016/j.cgh.2018.10.034. Epub 2018 Oct 26. Clin Gastroenterol Hepatol. 2019. PMID: 30613001
-
[Study of the effects of long-term outcomes of autologous peripheral blood stem cell reinfusion in patients with decompensated cirrhosis].Zhonghua Gan Zang Bing Za Zhi. 2022 Mar 20;30(3):279-284. doi: 10.3760/cma.j.cn501113-20220228-00091. Zhonghua Gan Zang Bing Za Zhi. 2022. PMID: 35462483 Chinese.
-
Allogeneic and autologous stem-cell transplantation in advanced Ewing tumors. An update after long-term follow-up from two centers of the European Intergroup study EICESS. Stem-Cell Transplant Programs at Düsseldorf University Medical Center, Germany and St. Anna Kinderspital, Vienna, Austria.Ann Oncol. 2000 Nov;11(11):1451-62. doi: 10.1023/a:1026539908115. Ann Oncol. 2000. PMID: 11142486 Review.
-
Hematopoietic stem cell graft manipulation as a mechanism of immunotherapy.Int Immunopharmacol. 2003 Aug;3(8):1121-43. doi: 10.1016/S1567-5769(03)00014-6. Int Immunopharmacol. 2003. PMID: 12860168 Review.
Cited by
-
Serous Membrane Detachment with Ultrasonic Homogenizer Improves Engraftment of Fetal Liver to Liver Surface in a Rat Model of Cirrhosis.Int J Mol Sci. 2021 Oct 27;22(21):11589. doi: 10.3390/ijms222111589. Int J Mol Sci. 2021. PMID: 34769019 Free PMC article.
-
Recellularization of Native Tissue Derived Acellular Scaffolds with Mesenchymal Stem Cells.Cells. 2021 Jul 15;10(7):1787. doi: 10.3390/cells10071787. Cells. 2021. PMID: 34359955 Free PMC article. Review.
-
Amniotic fluid characteristics and its application in stem cell therapy: A review.Int J Reprod Biomed. 2022 Sep 6;20(8):627-643. doi: 10.18502/ijrm.v20i8.11752. eCollection 2022 Aug. Int J Reprod Biomed. 2022. PMID: 36313262 Free PMC article. Review.
-
Long-term Outcome of Autologous Hematopoietic Stem Cell Infusion in Cirrhosis: Waning Effect over Time.J Clin Transl Hepatol. 2020 Dec 28;8(4):385-390. doi: 10.14218/JCTH.2020.00052. Epub 2020 Oct 14. J Clin Transl Hepatol. 2020. PMID: 33447521 Free PMC article.
-
Transplantation of Human Urine-Derived Stem Cells Ameliorates Erectile Function and Cavernosal Endothelial Function by Promoting Autophagy of Corpus Cavernosal Endothelial Cells in Diabetic Erectile Dysfunction Rats.Stem Cells Int. 2019 Sep 9;2019:2168709. doi: 10.1155/2019/2168709. eCollection 2019. Stem Cells Int. 2019. PMID: 31582984 Free PMC article.
References
-
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. - PubMed
-
- Poordad FF. Presentation and complications associated with cirrhosis of the liver. Curr Med Res Opin. 2015;31:925–937. - PubMed
-
- Lerschmacher O, Koch A, Streetz K, Trautwein C, Tacke F. [Management of decompensated liver cirrhosis in the intensive care unit] Med Klin Intensivmed Notfmed. 2013;108:646–656. - PubMed
-
- El-Masry M, Puig CA, Saab S. Recurrence of non-viral liver disease after orthotopic liver transplantation. Liver Int. 2011;31:291–302. - PubMed
-
- Abdeldayem HM, Allam NA, Salah E, Mostafa Aziz A, Kashkoush S, Adawy NM, Gad H, Helmy A. Moral and ethical issues in living-donor liver transplant in Egypt. Exp Clin Transplant. 2009;7:18–24. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous