Reformulated mineral trioxide aggregate components and the assessments for use as future dental regenerative cements
- PMID: 30397430
- PMCID: PMC6207958
- DOI: 10.1177/2041731418807396
Reformulated mineral trioxide aggregate components and the assessments for use as future dental regenerative cements
Abstract
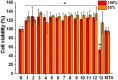
Mineral trioxide aggregate, which comprises three major inorganic components, namely, tricalcium silicate (C3S), dicalcium silicate (C2S), and tricalcium aluminate (C3A), is promising regenerative cement for dentistry. While mineral trioxide aggregate has been successfully applied in retrograde filling, the exact role of each component in the mineral trioxide aggregate system is largely unexplored. In this study, we individually synthesized the three components, namely, C3S, C2A, and C3A, and then mixed them to achieve various compositions (a total of 14 compositions including those similar to mineral trioxide aggregate). All powders were fabricated to obtain high purity. The setting reaction of all cement compositions was within 40 min, which is shorter than for commercial mineral trioxide aggregate (~150 min). Over time, the pH of the composed cements initially showed an abrupt increase and then plateaued (pH 10-12), which is a typical behavior of mineral trioxide aggregate. The compression and tensile strength of the composed cements increased (2-4 times the initial values) with time for up to 21 days in an aqueous medium, the degree to which largely depended on the composition. The cell viability test with rat mesenchymal stem cells revealed no toxicity for any composition except C3A, which contained aluminum. To confirm the in vivo biological response, cement was retro-filled into an extracted rat tooth and the complex was re-implanted. Four weeks post-operation, histological assessments revealed that C3A caused significant tissue toxicity, while good tissue compatibility was observed with the other compositions. Taken together, these results reveal that of the three major constituents of mineral trioxide aggregate, C3A generated significant toxicity in vitro and in vivo, although it accelerated setting time. This study highlights the need for careful consideration with regard to the composition of mineral trioxide aggregate, and if possible (when other properties are satisfactory), the C3A component should be avoided, which can be achieved by the mixture of individual components.
Keywords: Mineral trioxide aggregate; dicalcium silicate; intentional replantation; tricalcium aluminate; tricalcium silicate.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
-
- Gandolfi MG, Siboni F, Prati C. Chemical–physical properties of pure dicalcium silicate, tricalcium silicate and MM-MTA. Dent Mater 2014; 30: e72–e73.
-
- Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod 2009; 35(6): 777–790. - PubMed
-
- Taha NA, Ahmad MB, Ghanim A. Assessment of mineral trioxide aggregate pulpotomy in mature permanent teeth with carious exposures. Int Endod J 2017; 50(2): 117–125. - PubMed
LinkOut - more resources
Full Text Sources

