Magnetic ion channel activation of TREK1 in human mesenchymal stem cells using nanoparticles promotes osteogenesis in surrounding cells
- PMID: 30397432
- PMCID: PMC6207961
- DOI: 10.1177/2041731418808695
Magnetic ion channel activation of TREK1 in human mesenchymal stem cells using nanoparticles promotes osteogenesis in surrounding cells
Abstract
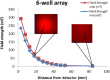
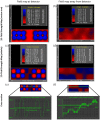
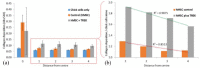
Magnetic ion channel activation technology uses superparamagnetic nanoparticles conjugated with targeting antibodies to apply mechanical force directly to stretch-activated ion channels on the cell surface, stimulating mechanotransduction and downstream processes. This technique has been reported to promote differentiation towards musculoskeletal cell types and enhance mineralisation. Previous studies have shown how mesenchymal stem cells injected into a pre-mineralised environment such as a foetal chick epiphysis, results in large-scale osteogenesis at the target site. However, the relative contributions of stem cells and surrounding host tissue has not been resolved, that is, are the mesenchymal stem cells solely responsible for the observed mineralisation or do mechanically stimulated mesenchymal stem cells also promote a host-tissue mineralisation response? To address this, we established a novel two-dimensional co-culture assay, which indicated that magnetic ion channel activation stimulation of human mesenchymal stem cells does not significantly promote migration but does enhance collagen deposition and mineralisation in the surrounding cells. We conclude that one of the important functions of injected human mesenchymal stem cells is to release biological factors (e.g., cytokines and microvesicles) which guide the surrounding tissue response, and that remote control of this signalling process using magnetic ion channel activation technology may be a useful way to both drive and regulate tissue regeneration and healing.
Keywords: Magnetic nanoparticles; mesenchymal stem cell; paracrine; stretch-activated ion channel; tissue engineering.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Figures




References
-
- Henstock JR, Hu B, Markides H, et al. Applications of magnetic nanoparticles in tissue engineering and regenerative medicine. In: Dobson J. (ed.) Nanomagnetic actuation of cell surface receptors: basic principles and applications. Boca Raton: Taylor & Francis, 2018.
-
- Henstock JR, El Haj AJ. Remotely controlled magnetic nanoparticles for bone regeneration. Regen Med 2015; 10: 377–380. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

