Emerging implications of policies on malaria treatment: genetic changes in the Pfmdr-1 gene affecting susceptibility to artemether-lumefantrine and artesunate-amodiaquine in Africa
- PMID: 30397515
- PMCID: PMC6202998
- DOI: 10.1136/bmjgh-2018-000999
Emerging implications of policies on malaria treatment: genetic changes in the Pfmdr-1 gene affecting susceptibility to artemether-lumefantrine and artesunate-amodiaquine in Africa
Abstract
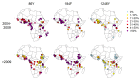
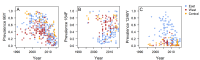
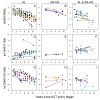
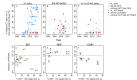
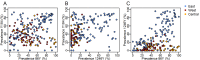
Artemether-lumefantrine (AL) and artesunate-amodiaquine (AS-AQ) are the most commonly used artemisinin-based combination therapies (ACT) for treatment of Plasmodium falciparum in Africa. Both treatments remain efficacious, but single nucleotide polymorphisms (SNPs) in the Plasmodium falciparum multidrug resistance 1 (Pfmdr1) gene may compromise sensitivity. AL and AS-AQ exert opposing selective pressures: parasites with genotype 86Y, Y184 and 1246Y are partially resistant to AS-AQ treatment, while N86, 184 F and D1246 are favoured by AL treatment. Through a systematic review, we identified 397 surveys measuring the prevalence of Pfmdr1 polymorphisms at positions 86 184 or 1246 in 30 countries in Africa. Temporal trends in SNP frequencies after introduction of AL or AS-AQ as first-line treatment were analysed in 32 locations, and selection coefficients estimated. We examined associations between antimalarial policies, consumption, transmission intensity and rate of SNP selection. 1246Y frequency decreased on average more rapidly in locations where national policy recommended AL (median selection coefficient(s) of -0.083), compared with policies of AS-AQ or both AL and AS-AQ (median s=-0.035 and 0.021, p<0.001 respectively). 86Y frequency declined markedly after ACT policy introduction, with a borderline significant trend for a more rapid decline in countries with AL policies (p=0.055). However, these trends could also be explained by a difference in initial SNP frequencies at the time of ACT introduction. There were non-significant trends for faster selection of N86 and D1246 in areas with higher AL consumption and no trend with transmission intensity. Recorded consumption of AS-AQ was low in the locations and times Pfmdr1 data were collected. SNP trends in countries with AL policies suggest a broad increase in sensitivity of parasites to AS-AQ, by 7-10 years after AL introduction. Observed rates of selection have implications for planning strategies to cycle drugs or use multiple first-line therapies to maintain drug efficacy.
Keywords: epidemiology; malaria; parasitology; public health; systematic review.
Conflict of interest statement
Competing interests: LCO declares grant funding from the WHO, the Bill and Melinda Gates Foundation and Medicines for Malaria Venture (MMV) and has received a consultancy contract in the past 3 years from WHO.
Figures
References
-
- Farooq U, Mahajan RC. Drug resistance in malaria. J Vector Borne Dis 2004;41:45–53. - PubMed
-
- World Health Organization , 2017. World malaria report 2017. http://www.who.int/malaria/publications/world-malaria-report-2017/en/ (accessed 16 Mar 2018).
-
- World Health Organization WHO Guidelines for the treatment of malaria. 3rd edn, 2015. - PubMed
-
- Venkatesan M, Gadalla NB, Stepniewska K, et al. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. Am J Trop Med Hyg 2014;91:833–43. 10.4269/ajtmh.14-0031 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
