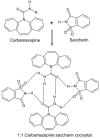
Recent advances in improving oral drug bioavailability by cocrystals
- PMID: 30397585
- PMCID: PMC6209825
- DOI: 10.15171/bi.2018.33
Recent advances in improving oral drug bioavailability by cocrystals
Abstract
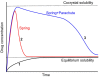
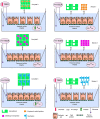
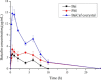
Introduction : Oral drug delivery is the most favored route of drug administration. However, poor oral bioavailability is one of the leading reasons for insufficient clinical efficacy. Improving oral absorption of drugs with low water solubility and/or low intestinal membrane permeability is an active field of research. Cocrystallization of drugs with appropriate coformers is a promising approach for enhancing oral bioavailability. Methods : In the present review, we have focused on recent advances that have been made in improving oral absorption through cocrystallization. The covered areas include supersaturation and its importance on oral absorption of cocrystals, permeability of cocrystals through membranes, drug-coformer pharmacokinetic (PK) interactions, conducting in vivo-in vitro correlations for cocrystals. Additionally, a discussion has been made on the integration of nanocrystal technology with supramolecular design. Marketed cocrystal products and PK studies in human subjects are also reported. Results : Considering supersaturation and consequent precipitation properties is necessary when evaluating dissolution and bioavailability of cocrystals. Appropriate excipients should be included to control precipitation kinetics and to capture solubility advantage of cocrystals. Beside to solubility, cocrystals may modify membrane permeability of drugs. Therefore, cocrystals can find applications in improving oral bioavailability of poorly permeable drugs. It has been shown that cocrystals may interrupt cellular integrity of cellular monolayers which can raise toxicity concerns. Some of coformers may interact with intestinal absorption of drugs through changing intestinal blood flow, metabolism and inhibiting efflux pumps. Therefore, caution should be taken into account when conducting bioavailability studies. Nanosized cocrystals have shown a high potential towards improving absorption of poorly soluble drugs. Conclusions : Cocrystals have found their way from the proof-of-principle stage to the clinic. Up to now, at least two cocrystal products have gained approval from regulatory bodies. However, there are remaining challenges on safety, predicting in vivo behavior and revealing real potential of cocrystals in the human.
Keywords: Bioavailability; Cocrystal; Oral absorption; Permeability; Pharmacokinetic; Poorly soluble drug.
Figures
Similar articles
-
Recent Advances on the Biological Study of Pharmaceutical Cocrystals.AAPS PharmSciTech. 2022 Nov 17;23(8):303. doi: 10.1208/s12249-022-02451-1. AAPS PharmSciTech. 2022. PMID: 36396736 Review.
-
Effect of Coformer Selection on In Vitro and In Vivo Performance of Adefovir Dipivoxil Cocrystals.Pharm Res. 2021 Oct;38(10):1777-1791. doi: 10.1007/s11095-021-03116-7. Epub 2021 Nov 2. Pharm Res. 2021. PMID: 34729701
-
Engineering Cocrystals of PoorlyWater-Soluble Drugs to Enhance Dissolution in Aqueous Medium.Pharmaceutics. 2018 Jul 31;10(3):108. doi: 10.3390/pharmaceutics10030108. Pharmaceutics. 2018. PMID: 30065221 Free PMC article. Review.
-
In Vitro-In Vivo Correlation in Cocrystal Dissolution: Consideration of Drug Release Profiles Based on Coformer Dissolution and Absorption Behavior.Mol Pharm. 2021 Nov 1;18(11):4122-4130. doi: 10.1021/acs.molpharmaceut.1c00537. Epub 2021 Oct 7. Mol Pharm. 2021. PMID: 34618448
-
Novel Aceclofenac-l-Cystine and Aceclofenac-Urea Cocrystals with Enhanced Oral Bioavailability.Curr Drug Deliv. 2021;18(8):1174-1181. doi: 10.2174/1567201818666210218103303. Curr Drug Deliv. 2021. PMID: 33602085
Cited by
-
Novel Purine Alkaloid Cocrystals with Trimesic and Hemimellitic Acids as Coformers: Synthetic Approach and Supramolecular Analysis.Cryst Growth Des. 2021 Jan 6;21(1):396-413. doi: 10.1021/acs.cgd.0c01242. Epub 2020 Dec 28. Cryst Growth Des. 2021. PMID: 36466627 Free PMC article.
-
Efficacy and Safety of Fixed-Dose Combinations for Pain in Older Adults.Drugs Aging. 2024 Nov;41(11):873-879. doi: 10.1007/s40266-024-01156-3. Epub 2024 Oct 25. Drugs Aging. 2024. PMID: 39453601 Review.
-
Engineering of Long-Term Stable Transparent Nanoemulsion Using High-Gravity Rotating Packed Bed for Oral Drug Delivery.Int J Nanomedicine. 2020 Apr 9;15:2391-2402. doi: 10.2147/IJN.S238788. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32308390 Free PMC article.
-
A Population Pharmacokinetic Study to Compare a Novel Empagliflozin L-Proline Formulation with Its Conventional Formulation in Healthy Subjects.Pharmaceuticals (Basel). 2024 Apr 18;17(4):522. doi: 10.3390/ph17040522. Pharmaceuticals (Basel). 2024. PMID: 38675482 Free PMC article.
-
Recent Advances on the Biological Study of Pharmaceutical Cocrystals.AAPS PharmSciTech. 2022 Nov 17;23(8):303. doi: 10.1208/s12249-022-02451-1. AAPS PharmSciTech. 2022. PMID: 36396736 Review.
References
-
- Food, Administration D. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. Food and Drug Administration, Washington, DC 2003.
-
- Chan OH, Stewart BH. Physicochemical and drug-delivery considerations for oral drug bioavailability. Drug Discov Today. 1996;1:461–73. doi: 10.1016/1359-6446(96)10039-8. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources