Perturbations of Neuron-Restrictive Silencing Factor Modulate Corticotropin-Releasing Hormone Gene Expression in the Human Cell Line BeWo
- PMID: 30397598
- PMCID: PMC6206961
- DOI: 10.1159/000492635
Perturbations of Neuron-Restrictive Silencing Factor Modulate Corticotropin-Releasing Hormone Gene Expression in the Human Cell Line BeWo
Abstract
Stress exacerbates disease, and understanding its molecular mechanisms is crucial to the development of novel therapeutic interventions to combat stress-related disorders. The driver of the stress response in the hypothalamic-pituitary-adrenal axis (HPA) is corticotropin-releasing hormone (CRH), a neuropeptide synthesized in the paraventricular nucleus of the hypothalamus. Evidence supports that CRH expression is epigenetically modified at the molecular level by environmental stimuli, causing changes in the stress response. This effect is mediated by a concert of factors that translate environmental change into alterations in gene expression. An important regulator and epigenetic modulator of CRH expression is neuron-restrictive silencing factor (NRSF). Previously, our lab identified numerous splice variants of NRSF that are specific to humans and predictive of differential regulatory effects of NRSF variants on targeted gene expression. The human cell line BeWo has endogenous CRH and NRSF expression providing an in vitro model system. Here, we show that manipulation of NRSF expression through siRNA technology, overexpression by plasmid vectors, and direct cAMP induction that CRH expression is linked to changes in NRSF expression. Accordingly, this epigenetic regulatory pathway in humans might be a critical mechanism involved in the regulation of the stress response.
Keywords: Corticotropin-releasing hormone; Neuron-restrictive silencing factor; Stress regulation.
Figures
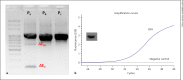

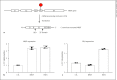

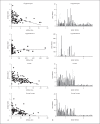
References
-
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374. - PubMed
-
- McEwen BS. In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann NY Acad Sci. 2016;1373:56–64. - PubMed
-
- De Souza EB, Battaglia G. Corticotropin-releasing hormone (CRH) receptors in brain. Adv Exp Med Biol. 1988;245:123–136. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

