Multichannel optrodes for photonic stimulation
- PMID: 30397630
- PMCID: PMC6197865
- DOI: 10.1117/1.NPh.5.4.045002
Multichannel optrodes for photonic stimulation
Abstract
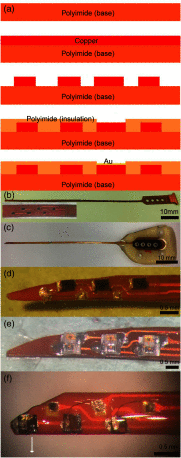
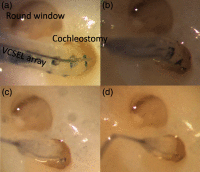
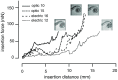
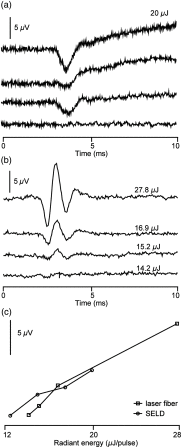
An emerging method in the field of neural stimulation is the use of photons to activate neurons. The possible advantage of optical stimulation over electrical is attributable to its spatially selective activation of small neuron populations, which is promising in generating superior spatial resolution in neural interfaces. Two principal methods are explored for cochlear prostheses: direct stimulation of nerves with infrared light and optogenetics. This paper discusses basic requirements for developing a light delivery system (LDS) for the cochlea and provides examples for building such devices. The proposed device relies on small optical sources, which are assembled in an array to be inserted into the cochlea. The mechanical properties, the biocompatibility, and the efficacy of optrodes have been tested in animal models. The force required to insert optrodes into a model of the human scala tympani was comparable to insertion forces obtained for contemporary cochlear implant electrodes. Side-emitting diodes are powerful enough to evoke auditory responses in guinea pigs. Chronic implantation of the LDS did not elevate auditory brainstem responses over 26 weeks.
Keywords: cochlear implant; infrared neural stimulation; laser; light delivery system; optrode.
Figures