Transistor in a tube: A route to three-dimensional bioelectronics
- PMID: 30397642
- PMCID: PMC6203411
- DOI: 10.1126/sciadv.aat4253
Transistor in a tube: A route to three-dimensional bioelectronics
Abstract
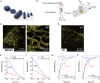
Advances in three-dimensional (3D) cell culture materials and techniques, which more accurately mimic in vivo systems to study biological phenomena, have fostered the development of organ and tissue models. While sophisticated 3D tissues can be generated, technology that can accurately assess the functionality of these complex models in a high-throughput and dynamic manner is not well adapted. Here, we present an organic bioelectronic device based on a conducting polymer scaffold integrated into an electrochemical transistor configuration. This platform supports the dual purpose of enabling 3D cell culture growth and real-time monitoring of the adhesion and growth of cells. We have adapted our system to a 3D tubular geometry facilitating free flow of nutrients, given its relevance in a variety of biological tissues (e.g., vascular, gastrointestinal, and kidney) and processes (e.g., blood flow). This biomimetic transistor in a tube does not require photolithography methods for preparation, allowing facile adaptation to the purpose. We demonstrate that epithelial and fibroblast cells grow readily and form tissue-like architectures within the conducting polymer scaffold that constitutes the channel of the transistor. The process of tissue formation inside the conducting polymer channel gradually modulates the transistor characteristics. Correlating the real-time changes in the steady-state characteristics of the transistor with the growth of the cultured tissue, we extract valuable insights regarding the transients of tissue formation. Our biomimetic platform enabling label-free, dynamic, and in situ measurements illustrates the potential for real-time monitoring of 3D cell culture and compatibility for use in long-term organ-on-chip platforms.
Figures



References
-
- Spira M. E., Hai A., Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 8, 83–94 (2013). - PubMed
-
- Heer F., Franks W., Blau A., Taschini S., Ziegler C., Hierlemann A., Baltes H., CMOS microelectrode array for the monitoring of electrogenic cells. Biosens. Bioelectron. 20, 358–366 (2004). - PubMed
-
- Fromherz P., Offenhäusser A., Vetter T., Weis J., A neuron-silicon junction: A Retzius cell of the leech on an insulated-gate field-effect transistor. Science 252, 1290–1293 (1991). - PubMed
-
- Rivnay J., Inal S., Salleo A., Owens R. M., Berggren M., Malliaras G. G., Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

